|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
ClCH2CH2CH2CH3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in 1-Chloro-n-Butane TT
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine nqcc's in 1-chloro-n-butane TT was made on approximate equilibrium structures given by MP2 optimization in conjunction with Pople-type bases, with empirical correction for the CH, C-C, and CCl bond lengths (~ re).
These given in Tables 1 and 2. Structure parameters are given in
Table 3, rotational constants in Table 4. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 2,
subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. Øz,CCl (degrees) is the angle between the principal z-axis of the nqcc tensor and the CCl bond axis. ETA = (Xxx - Xyy)/Xzz. |
|
|
RSD is the calibration residual standard deviation of
the B1LYP/TZV(3df,2p) model for calculation of the chlorine nqcc's,
which may be taken as an estimate of the uncertainty in the calculated
nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1.
35Cl nqcc's in 1-chloro-n-butane TT (MHz). Calculation was made on the ~ re structure, with interatomic angles given by (1) MP2/6-311+G(d,p) and (2) MP2/6-311+G(2d,p) optimizations. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc/~ re(1)
|
|
Calc/~ re(2) |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
42.93 |
- |
43.80 |
|
|
|
Xbb |
|
6.98 |
|
7.89 |
|
|
|
Xcc |
|
35.95 |
|
35.90 |
|
|
|
|Xab| |
|
47.20 |
|
46.47 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
35.41 |
|
35.22 |
|
|
|
|
Xyy |
|
35.95 |
|
35.90 |
|
|
|
|
Xzz |
- |
71.36 |
- |
71.13 |
|
|
|
|
ETA |
|
0.007 |
|
0.009 |
|
|
|
|
Øz,a |
|
31.06 |
|
30.46 |
|
|
|
|
Øa,CCl |
|
30.15 |
|
29.72 |
|
|
|
|
Øz,CCl |
|
0.92 |
|
0.74 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2.
37Cl nqcc's in 1-chloro-n-butane TT (MHz). Calculation was made on the ~ re structure, with interatomic angles given by (1) MP2/6-311+G(d,p) and (2) MP2/6-311+G(2d,p) optimizations. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc/~ re(1)
|
|
Calc/~ re(2) |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
34.01 |
- |
34.69 |
|
|
|
Xbb |
|
5.68 |
|
6.40 |
|
|
|
Xcc |
|
28.33 |
|
28.30 |
|
|
|
|Xab| |
|
37.10 |
|
36.53 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
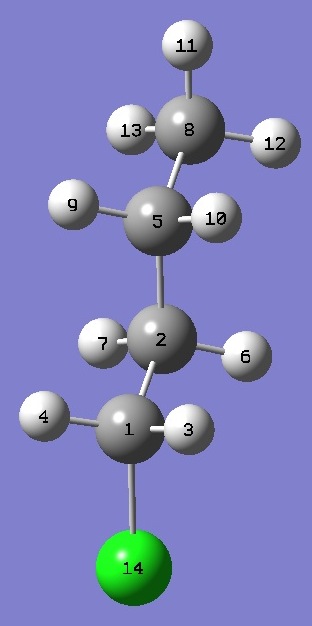
| Table 3. 1-Chloro-n-Butane TT (Cs). Heavy atom structure parameters, ~ re(1) and ~ re(2) (Å
and degrees). Complete structures are given here in Z-matrix format. |
| ~ re(1) is ~ re bond lengths with MP2/6-311+G(d,p) interatomic angles, ~ re(2) is ~ re bond lengths with MP2/6-311+G(2d,p) angles. |
| |
|
|
|
|
|
~ re(1) |
~ re(2) |
|
|
|
| ClC(1) |
1.7863 |
1.7863 |
| C(1)C(2) |
1.5111 |
1.5111 |
| C(2)C(5) |
1.5214 |
1.5214 |
| C(5)C(8) |
1.5209 |
1.5209 |
| ClC(1)C(2) |
111.35 |
111.67 |
| C(1)C(2)C(5) |
111.72 |
111.17 |
| C(2)C(5)C(8) |
112.09 |
111.98 |
| |
|
|
| Click on image to enlarge. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
| Table 4. Rotational Constants (MHz). 35Cl Species. |
|
|
|
|
|
|
|
~ re(1) |
~ re(2) |
Expt. [1] |
|
|
|
|
|
|
A |
16 992.3 |
17 114.9 |
16 867(5) |
|
B |
1 334.2 |
1 333.0 |
1 321.148(4) |
|
C |
1 276.2 |
1 275.7 |
1 263.943(4) |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Substitution coordinates of the Cl
atom were determined [1]: These are (| a |, | b |, | c |) =
(2.1418, 0.2044, 0 Å). On the ~ re(1) structure, (| a |, | b |, | c |) = (2.1328, 0.2073, 0 Å); and on the ~ re(2) structure, = (2.1349, 0.2034, 0 Å). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] S.Melandri, P.G.Favero, D.Damiani, W.Caminati, and L.B.Favero, J.Chem.Soc. Faraday Trans. 90,2183(1994).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1-Chloro-n-Butane GT |
1-Chloro-n-Butane TG |
|
|
|
1-Chloro-n-Butane GG |
1-Chloro-n-Butane GG' |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1ClnButane_TT.html |
|
|
|
|
|
|
Last
Modified 14 Jan 2008 |
|
|
|
|
|
|
|
|
|
|

