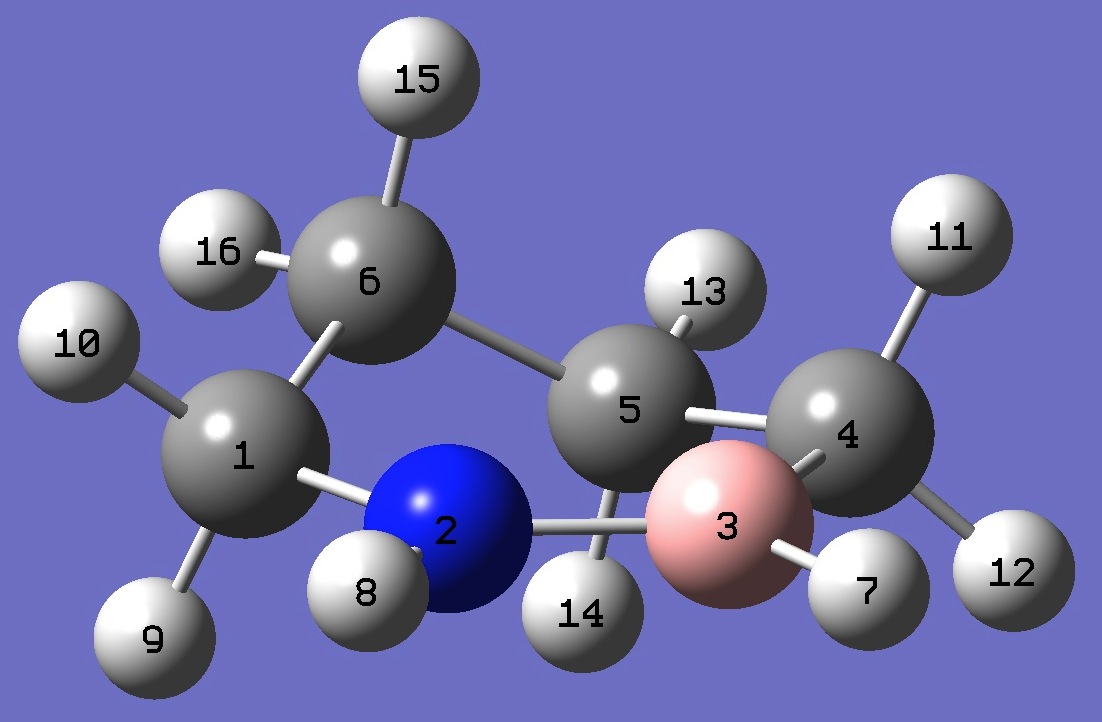
| |
||||||||
| Table 1. 11B nqcc's in 1,2-BN Cyclohexene (MHz). Calculation was made on the (1) B3P86/6-311G(3d,3p) and (2) mPW1PW91/6-311G(3d,3p) optimized structures. | ||||||||
| |
||||||||
| Calc. (1) |
Calc. (2) | Expt. [1] | ||||||
| |
||||||||
| Xaa | - |
1.815 |
- |
1.819 |
- |
1.7989(52) * |
||
| Xbb | - |
1.833 |
- |
1.828 |
- |
1.8145(64) * |
||
| Xcc | 3.648 |
3.647 |
3.6135(64) * |
|||||
| Xab | 0.362 |
0.359 |
||||||
| Xac | 0.225 |
0.224 |
||||||
| Xbc | 0.456 |
0.455 |
||||||
| RMS | 0.024 (1.0 %) |
0.024 (0.99 %) |
||||||
| RSD | 0.046 (2.1 %) |
0.046 (2.1 %) | ||||||
| Xxx | - |
1.506 |
- |
1.509 |
||||
| Xyy | - |
2.191 |
- |
2.187 |
||||
| Xzz | 3.697 |
3.696 |
||||||
| ETA | 0.185 |
0.184 |
||||||
| Øc,z * | 5.56 |
5.55 |
||||||
| |
||||||||
| Table 2. 14N nqcc's in 1,2-BN Cyclohexene (MHz). Calculation was made on the (1) B3P86/6-311G(3d,3p) and (2) mPW1PW91/6-311G(3d,3p) optimized structures. | ||||||||
| |
||||||||
| Calc. (1) |
Calc. (2) | Expt. [1] | ||||||
| |
||||||||
| Xaa | 0.430 |
0.432 |
0.3687(41) * |
|||||
| Xbb | 2.140 |
2.130 |
2.1295(36) * |
|||||
| Xcc | - |
2.570 |
- |
2.562 |
- |
2.4981(36) * |
||
| Xab | - |
0.921 |
- |
0.923 |
||||
| Xac | - |
0.192 |
- |
0.191 |
||||
| Xbc | - |
0.273 |
- |
0.273 |
||||
| RMS | 0.055 (3.3 %) |
0.052 (3.1 %) |
||||||
| RSD | 0.030 (1.3 %) | 0.030 (1.3 %) | ||||||
| Xxx | 0.059 |
0.058 |
||||||
| Xyy | 2.548 |
2.541 |
||||||
| Xzz | - |
2.607 |
- |
2.599 |
||||
| ETA | 0.955 |
0.956 |
||||||
| Øc,z * | 6.46 |
6.48 |
||||||
| |
||||||||
| Table 3. 10B and 14N nqcc's in 1,2-BN Cyclohexene (MHz). Calculation was made on the (1) B3P86/6-311G(3d,3p) and (2) mPW1PW91/6-311G(3d,3p) optimized structures. | ||||||||
| |
||||||||
| Calc. (1) |
Calc. (2) | Expt. [1] | ||||||
| |
||||||||
| Xaa(10B) | - |
3.764 |
- |
3.771 |
- |
3.7479(61) * |
||
| Xbb | - |
3.799 |
- |
3.790 |
- |
3.7323(80) * |
||
| Xcc | 7.563 |
7.561 |
7.4801(80) * |
|||||
| Xab | 0.751 |
0.745 |
||||||
| Xac | 0.465 |
0.465 |
||||||
| Xbc | 0.960 |
0.957 |
||||||
| RMS | 0.062 (1.2 %) |
0.059 (1.2 %) |
||||||
| RSD | 0.102 (2.1 %) |
0.102 (2.1 %) | ||||||
| Xaa(14N) | 0.430 |
0.432 |
0.3689(41) |
|||||
| Xbb | 2.139 |
2.130 |
2.1356(47) |
|||||
| Xcc | 2.570 |
2.562 |
- |
2.5044(47) |
||||
| Xac | - |
0.921 |
- |
0.923 |
||||
| Xac | - |
0.191 |
- |
0.191 |
||||
| Xbc | - |
0.279 |
- |
0.273 |
||||
| |
||||||||
| RMS |
0.052 (3.1 %) |
0.049 (3.0 %) |
||||||
| RSD |
0.030 (1.3 %) | 0.030 (1.3 %) | ||||||
| |
||||||||
| Table 3. 1,2-11B14N Cyclohexene. Rotational Constants (MHz). Calc = B3P86/6-311G(3d,3p) and mPW1PW91/6-311G(3d,3p) optimization. | ||||
| Calc /B3P86 |
Calc /mPW1PW91 |
Expt. [1] | ||
| A | 4739. |
4744. |
4702.0578(17) |
|
| B | 4395. |
4395. |
4360.3340(10) |
|
| C | 2508. |
2509. |
2494.4070(10) |
|