|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
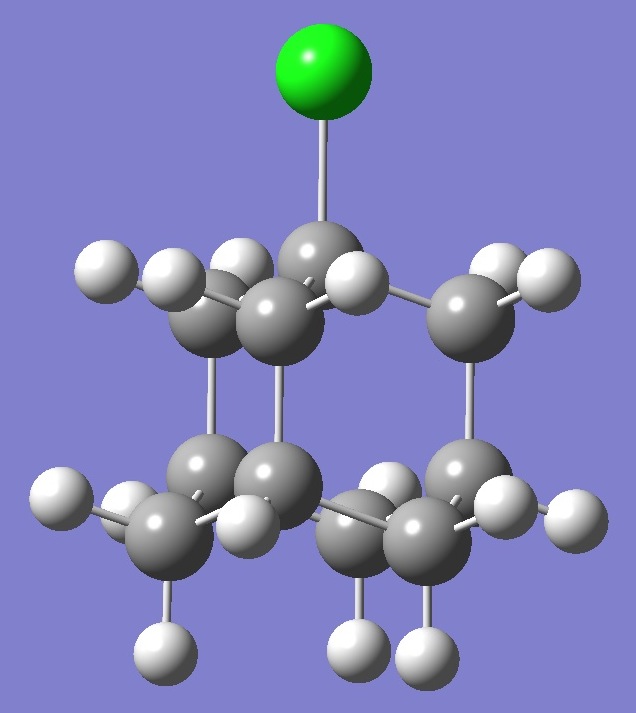
C10H15Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in
1-Chloroadamantane |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ellis et al. [1] measured the
chlorine nqcc's in 1-chloroadamantane
and determined a substitution CCl bond length of 1.8283 Å.
Chadwick et al [2] derived an effective structure with CC = 1.541
Å and CCl
= 1.790 Å under the assumption that all angles are tetrahedral
and
all CH bond lengths are 1.09 Å. |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine nqcc's
was made (i) on the structure of Chadwick et al., (ii) on this
structure but with the CCl bond distance of Ellis et
al., and (iii) on a structure given by MP2/6-311+G(d,p) optimization
with approximate equilibrium CCl and CH bond
lengths (~ re). Calculated and experimental nqcc's
are shown in Table 1. Structure parameters (iii) are shown in
Table
2. |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1.
Chlorine
nqcc's in 1-Chloroadamantane
(MHz). |
|
| |
|
|
|
|
|
|
|
|
|
Calc. (iii) MP2/6-311+G(d,p)
with ~ re(CCl) = 1.8000 Å. |
|
|
|
Calc. (i) Chadwick CCl = 1.790
Å. |
|
|
|
Calc. (ii) Ellis CCl = 1.8283
Å. |
|
|
|
|
|
|
|
|
|
| |
|
|
|
Calc. |
|
Expt. [1]
|
|
| |
|
|
|
|
|
|
|
|
35Cl |
eQq |
- |
67.03 (iii) |
- |
66.321(5) |
|
|
|
|
- |
67.50 (i) |
|
|
|
|
|
|
-
|
68.10 (ii) |
|
|
|
| |
|
|
|
|
|
|
|
|
37Cl |
eQq |
- |
52.83 (iii) |
- |
52.277(5) |
|
|
|
|
- |
53.20 (i) |
|
|
|
|
|
|
-
|
53.67 (ii) |
|
|
|
| |
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 2.
MP2/6-311+G(d,p) C-C bond lengths and CCC angles
(bridgehead) in
1-X-adamantane, where X = CN, Cl, Br, and I (Å and degrees).
For comparison, in adamantane optimized at the same level of
theory, all C-C = 1.5366
Å
and CCC = 109.36o. The
complete (iii)
structure of 1-chloroadamantane is given
here
in Z-matrix format. |
| |
|
|
|
|
|
X = CN |
Cl |
Br |
I |
|
|
|
|
|
| CC (bridgehead) |
1.5447 |
1.5283 |
1.5293 |
1.5309 |
| CCC (bridgehead) |
109.27 |
110.07 |
110.15 |
110.17 |
| CC (column) |
1.5355 |
1.5383 |
1.5397 |
1.5411 |
| CC (base) |
1.5360 |
1.5362 |
1.5356 |
1.5353 |
|
|
|
|
|
|
|
|
|
|
|
|
|
The rotational constant calculated on
the MP2/6-311+G(d,p) structure (iii) is 841.03 MHz, whereas the
experimental
rotational constant is |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] M.C.Ellis, A.C.Legon,
C.A.Rego, and D.J.Millen, J.Mol.Struct. (Theochem) 200,353(1989). |
|
|
[2] D.Chadwick, A.C.Legon, and
D.J.Millen, J.Chem.Soc.(A) 1116(1968). |
|
|
|
|
|
|
|
|
|
|
|
|
1-Fluoroadamantane: A.C.Legon,
J.Tizard, and Z.Kisiel, J.Mol.Struct. 612,83(2002). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1-Bromoadamantane |
1-Iodoadamantane |
1-Cyanoadamantane |
|
t-Butyl
Chloride |
t-Butyl Bromide |
t-Butyl Iododine |
|
t-Butyl Cyanide |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C10H15Cl.html |
|
|
|
|
|
|
Last
Modified 2 Nov 2009 |
|
|
|
|
|
|
|
|
|
|
