|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
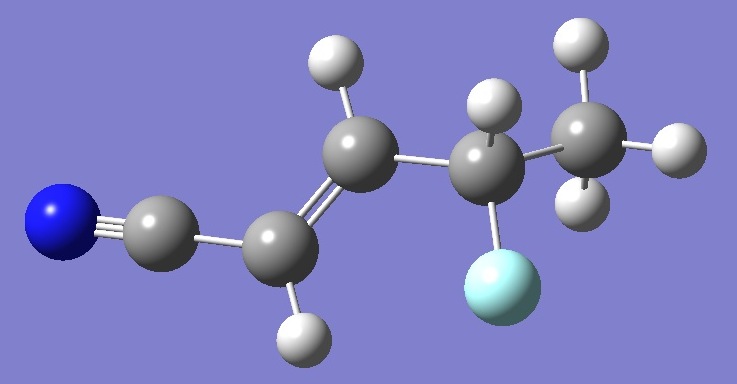
NCCHCHCHFCH3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in 1-Cyano-3-fluoro-but-1-ene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen nqcc's in 1-cyano-3-fluoro-but-1-ene (CF eclisped) were
determined by Kassi et al. [1]. Here, calculation was made of the nqcc's on a structure derived ab initio (see below). Calculated and experimental nqcc's
are compared in Table 1. Eigenvalues and eigenvectors of the nqcc
tensor are given in Table 2. Structure parameters are given in
Table 3, atomic coordinates in Table 4, rotational constants in Table 5.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, RMS is the root mean square difference
between calculated and experimental diagonal nqcc's (percentage of the
average of the magnitudes of the experimental nqcc's). RSD is the
calibration residual standard deviation of the B3PW91/6-311+G(df,pd) model
for calculation of the nitrogen nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
Subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. Ø (degrees) is the angle between its subscripted parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc's in 1-cyano-3-fluoro-but-1-ene (MHz). Calculation was made on the ab initio structure. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
3.860 |
- |
3.850(5) |
|
|
|
Xbb |
|
1.794 ? |
- |
2.068(2) |
|
|
|
Xcc |
|
2.066 ? |
|
5.918(3) |
|
|
|
Xab * |
|
1.521 |
|
|
|
|
|
Xac * |
- |
0.004 |
|
|
|
|
|
Xbc * |
- |
0.081 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
3.149 (80 %) |
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.025 |
|
|
|
|
|
Xyy |
|
2.219 |
|
|
|
|
|
Xzz |
- |
4.244 |
|
|
|
|
|
ETA |
|
0.046 |
|
|
|
|
|
Øz,CN |
|
0.58 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* The algebraic signs of the
off-diagonal components depend on the orientation of the molecule
with respect to a,b,c axes. Here, the algebraic signs correspond
to the atomic a,b,c coordinates given in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2. 1-cyano-3-fluoro-but-1-ene. Eigenvalues; Xii, i=x,y,z (MHz) and eigenvectors (direction cosines) of the nitrogen nqcc tensor. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
i = |
|
x |
|
y |
|
z |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xii |
|
2.025 |
|
2.219 |
- |
4.244 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
a |
|
0.1152 |
|
0.2154 |
|
0.9697 |
|
|
|
|
b |
|
0.4486 |
|
0.8597 |
- |
0.2443 |
|
|
|
|
c |
|
0.8863 |
- |
0.4631 |
- |
0.0024 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular Structure |
|
|
|
|
|
|
|
|
|
|
|
|
The structure shown below was
optimized at the MP2/6-31G(d,p) and MP2/6-311+G(d,p) levels of theory.
The optimized C-H, C-C, C=C, and C-F bond lengths were corrected
according to the methods of the Lille group [2-4]. Angles were determined by
freezing the corrected bond lengths and performing a partial optimization at the MP2/6-311+G(d,p) level of theory.
|
|
|
| |
|
|
| Table 3. Molecular structure parameters, ropt (Å
and degrees). |
| |
|
|
| Point Group: C1 |
C(1)C(2) |
1.5116 |
|
C(2)C(3) |
1.4912 |
|
C(3)C(4) |
1.3364 |
| C(4)C(5) |
1.4293 |
| C(5)N |
1.1578 |
| C(1)H(11) |
1.0888 |
| C(1)H(12) |
1.0891 |
| C(1)H(13) |
1.0900 |
|
C(2)H(10) |
1.0955 |
| Dihedral angles? See Z-Matrix. |
C(2)F |
1.3871 |
|
C(3)H(9) |
1.0854 |
|
C(4)H(8) |
1.0818 |
|
C(1)C(2)C(3) |
112.18 |
|
C(2)C(3)C(4) |
123.57 |
|
C(3)C(4)C(5) |
121.62 |
|
C(4)C(5)N |
179.28 |
|
C(2)C(1)H(11) |
109.97 |
|
C(2)C(1)H(12) |
109.80 |
|
C(2)C(1)H(13) |
110.23 |
|
C(3)C(2)H(10) |
109.08 |
|
C(3)C(2)F |
109.87 |
|
C(2)C(3)H(9) |
116.16 |
|
C(3)C(2)H(10) |
109.08 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
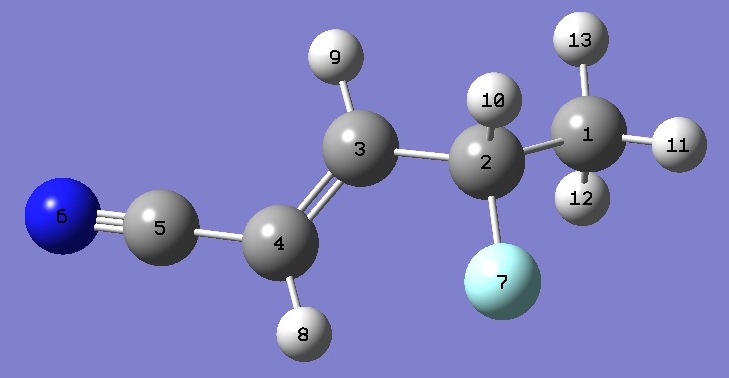
| Table 4. 1-cyano-3-fluoro-but-1-ene. Atomic coordinates, ropt. |
| (More figures are shown than are significant.) |
| |
|
|
|
|
|
|
|
|
|
|
a (Å) |
|
b (Å) |
|
c (Å) |
|
|
|
|
|
|
|
|
|
C |
- |
2.285386 |
- |
1.107261 |
- |
0.390584 |
|
C |
- |
1.483432 |
- |
0.112536 |
|
0.417083 |
|
C |
- |
0.025805 |
- |
0.427120 |
|
0.411012 |
|
C |
|
0.905342 |
|
0.394262 |
- |
0.083218 |
|
C |
|
2.290614 |
|
0.042245 |
- |
0.084677 |
|
N |
|
3.415857 |
- |
0.230282 |
- |
0.092383 |
|
F |
- |
1.694188 |
|
1.155981 |
- |
0.103007 |
|
H |
|
0.634131 |
|
1.355559 |
- |
0.498720 |
|
H |
|
0.258335 |
- |
1.386927 |
|
0.830686 |
|
H |
- |
1.840695 |
- |
0.090059 |
|
1.452447 |
|
H |
- |
3.341466 |
- |
0.843851 |
- |
0.362345 |
|
H |
- |
1.943508 |
- |
1.103789 |
- |
1.424627 |
|
H |
- |
2.162727 |
- |
2.110446 |
|
0.017672 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 5. 1-cyano-3-fluoro-but-1-ene. Rotational constants (MHz). |
| |
|
|
|
|
|
Calc. ropt |
Expt. [1] |
|
|
|
|
|
A |
7544.2 |
7493.404(1) |
|
B |
1217.3 |
1211.9831(2) |
|
C |
1101.6 |
1096.0908(1) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] S.Kassi, D.Grée, R.Grée, D.Duflot, D.Petitprez, and G.Wlodarczak, J.Mol.Spectrosc. 202,19(2000). |
|
|
[2] J.Demaison and G.Wlodarczak, Struct.Chem. 5,57(1994).
|
|
|
[3] J.Demaison, J.Cosléou, R.Bocquet, and A.G.Lesarri, J.Mol.Spectrosc. 167,400(1994).
|
|
|
[4]
R.M.Villamañan, W.D.Chen, G.Wlodarczak, J.Demaison, A.G.Lesarri,
J.C.López, and J.L.Alonso, J.Mol.Spectrosc. 171,223(1995). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CFB.html |
|
|
|
|
|
|
Last
Modified 28 Nov 2005 |
|
|
|
|
|
|
|
|
|
|

