|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(CH2)4-C(H)CN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Cyanocyclopentane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
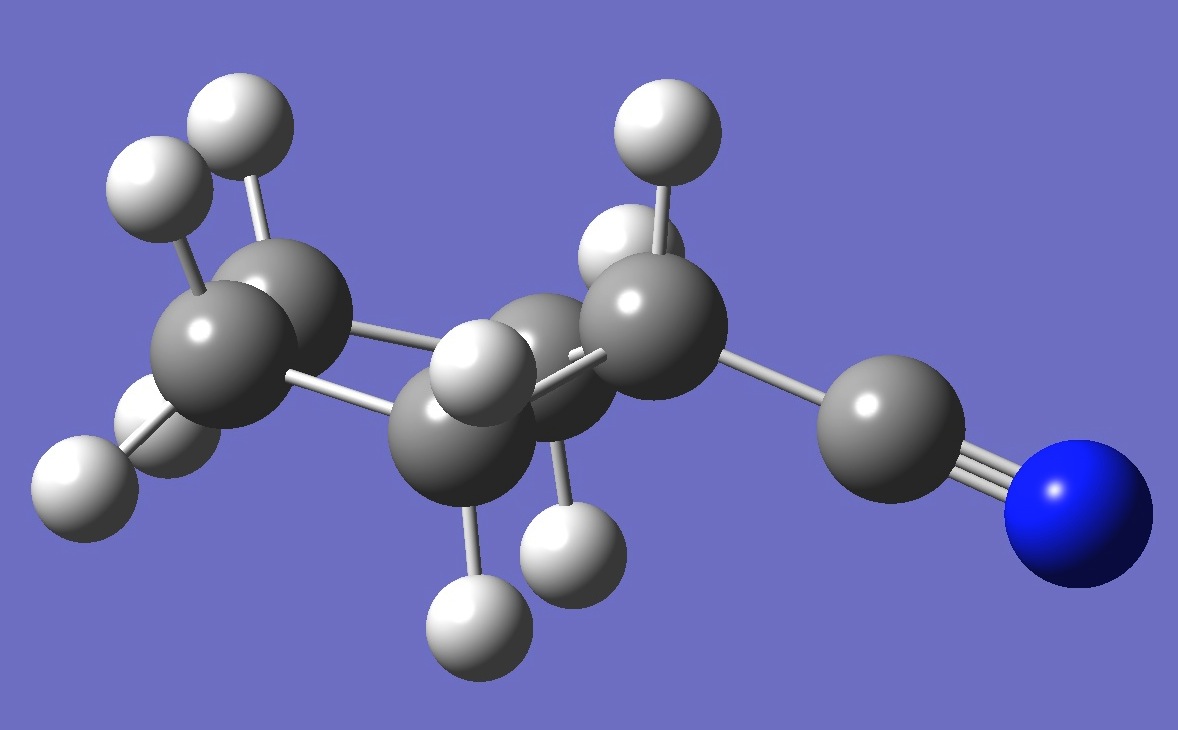
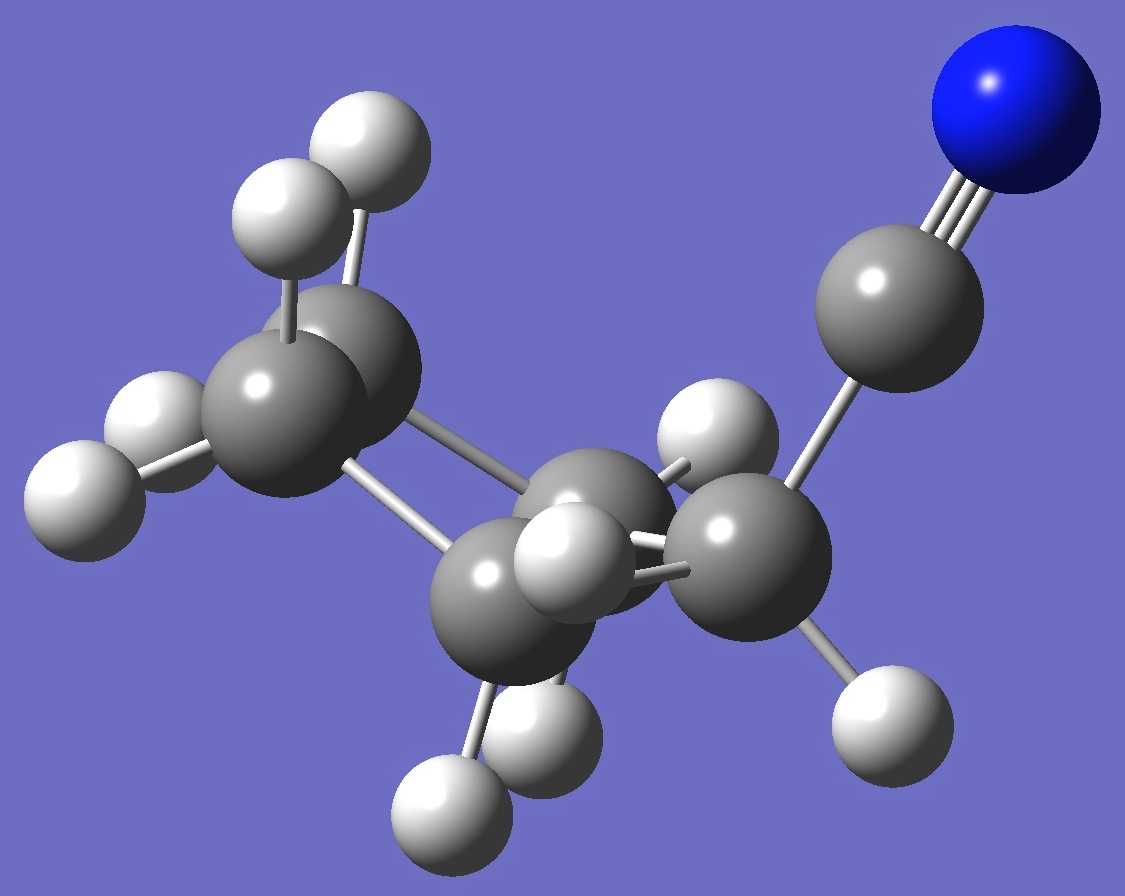
The microwave spectrum of both axial and equatorial cyanocyclopentane was observed and assigned by Choe and Harmony [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
For each conformer, calculation of the nitrogen nqcc
tensor was made on a molecular structure given by B3P86/6-31G(3d,3p) optimization. These
calculated nqcc's are given in Tables 1 and 2. Structure
parameters are given here in Z-Matrix format, rotational
constants and dipole moments in Table 3.
|
|
|
|
|
|
|
|
|
|
|
|
|
equatorial
|
|
|
axial
|
|
|
|
At the
|
|
|
|
B3P86/6-31G(3d,3p) |
|
|
level of theory,
|
|
|
Eeq < Eax
|
|
|
by 1.4 kJ/mol
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz.
Ø (degrees) is the angle between its subscripted parameters.
|
|
|
RSD is the calibration residual standard deviation of
the B3PW91/6-311+G(df,pd) model for calculation of the nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc tensor in equatorial cyanocyclopentane (MHz). Calculation was made on
a molecular structure given by B3P86/6-31G(3d,3p) optimization.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc.
|
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
-
|
3.833
|
|
|
|
|
|
Xbb |
|
2.082
|
|
|
|
|
|
Xcc |
|
1.750
|
|
|
|
|
|
Xac
|
|
1.469
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.113
|
|
|
|
|
|
Xyy |
|
2.082
|
|
|
|
|
|
Xzz |
-
|
4.196
|
|
|
|
|
|
ETA |
-
|
0.0073
|
|
|
|
|
|
Øz,a |
|
13.87
|
|
|
|
|
|
Øa,CN |
|
13.92
|
|
|
|
|
|
Øz,CN |
|
0.05
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen
nqcc tensor in axial cyanocyclopentane (MHz). Calculation was made on
a molecular structure given by B3P86/6-31G(3d,3p) optimization.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc.
|
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
-
|
1.985
|
|
|
|
|
|
Xbb |
|
2.042
|
|
|
|
|
|
Xcc |
-
|
0.056
|
|
|
|
|
|
Xac
|
|
3.018
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.148
|
|
|
|
|
|
Xyy |
|
2.042
|
|
|
|
|
|
Xzz |
-
|
4.190
|
|
|
|
|
|
ETA |
-
|
0.025
|
|
|
|
|
|
Øz,a |
|
36.14
|
|
|
|
|
|
Øa,CN |
|
36.46
|
|
|
|
|
|
Øz,CN |
|
0.32
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3. Cyanocyclopentane, rotational constants (MHz) and dipole moments (D).
|
|
|
|
|
|
|
|
|
________equatorial_________ |
__________axial___________ |
|
|
Calc
|
Expt [1]
|
Calc
|
Expt [1]
|
|
|
|
|
|
|
|
A |
6374
|
6324.905
|
4359
|
4297.196
|
|
B |
1794
|
1790.937
|
2191
|
2210.245
|
|
C |
1498
|
1497.792
|
2028
|
2057.205
|
|
|
|
|
|
|
|
|µa|
|
4.47
|
|
3.74
|
|
|
|µc| |
0.72
|
|
2.03
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] J.-I.Choe and M.D.Harmony, J.Mol.Spectrosc 81,480(1980).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cyanoyclopropane
|
Cyanocyclobutane
|
|
|
|
|
Cyanocyclohexane
|
Chlorocyclopentane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH24CHCN.html |
|
|
|
|
|
|
Last
Modified 24 Jan 2014
|
|
|
|
|
|
|
|
|
|
|

