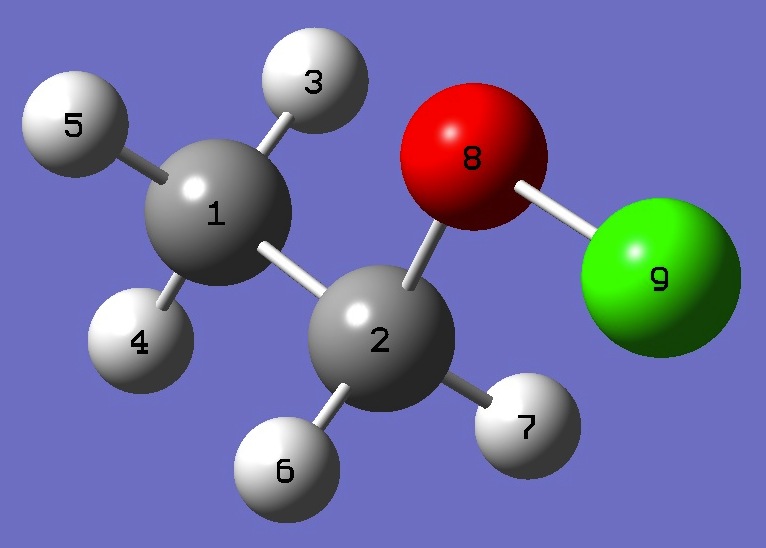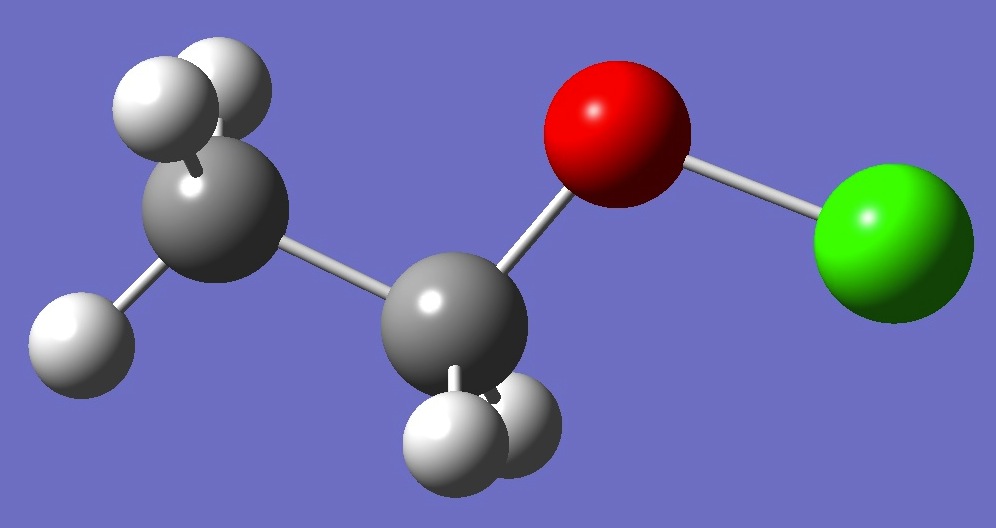
| |
||||||||
| Table 1. 35Cl nqcc's in CH3CH2OCl (MHz). Calculation was made
on the (1) MP2/cc-pVTZ and (2) MP2/cc-pVQZ ropt structures. |
||||||||
| |
||||||||
| Calc (1) | Calc (2) |
Expt [1] |
||||||
| |
||||||||
| Xaa | - |
95.28 |
- |
95.19 |
- |
93.96(62) |
||
| Xbb | 36.31 |
36.29 |
35.76(39) |
|||||
| Xcc | 58.97 |
58.89 |
58.20(28) |
|||||
| Xab | - |
57.63 |
- |
57.54 |
||||
| RMS |
0.94 (1.5 %) |
0.87 (1.4 %) |
||||||
| RSD | 0.49 (1.1 %) |
0.49 (1.1 %) | ||||||
| |
||||||||
| Xxx | 57.98 |
57.92 |
||||||
| Xyy | 58.97 | 58.89 |
||||||
| Xzz | - |
116.95 |
- |
116.81 |
||||
| ETA |
0.00839 |
0.00836 |
||||||
| Øz,a |
20.61 |
20.60 |
||||||
| Øa,OCl | 21.05 |
21.06 |
||||||
| Øz,OCl | 0.44 |
0.46 |
||||||
| |
||||||||
| |
||||||||
| Table 2. 37Cl nqcc's in CH3CH2OCl (MHz). Calculation was made
on the (1) MP2/cc-pVTZ and (2) MP2/cc-pVQZ ropt structures. |
||||||||
| |
||||||||
| Calc (1) | Calc (2) |
Expt [1] |
||||||
| |
||||||||
| Xaa | - |
75.19 |
- |
75.11 |
- |
67.4(25) |
||
| Xbb | 28.71 |
28.69 |
24.8(13) |
|||||
| Xcc | 46.48 |
46.42 |
42.6(12) |
|||||
| Xab | - |
45.32 |
- |
45.24 |
||||
| RMS |
5.50 (12.2 %) |
5.45 (12.1 %) |
||||||
| RSD | 0.44 (1.1 %) |
0.44 (1.1 %) | ||||||
| |
||||||||
| Table 4. CH3CH2OCl. Rotational Constants (MHz) and Dipole Moments * (D). ropt(1) = MP2/cc-pVTZ, ropt(2) = MP2/cc-pVQZ. | ||||
| |
||||
| ropt(1) | ropt(2) | Expt [1] |
||
| |
||||
| A |
31752.7 |
31895.0 |
31582.01(7) |
|
| B |
2708.7 |
2720.8 |
2691.084(10) |
|
| C |
2576.2 |
2588.0 |
2560.916(8) |
|
| |µa| | 1.58 |
1.58 |
1.632(10) |
|
| |µb| | 1.15 |
1.14 |
1.079(3) |
|
| |
||||
| * Calculated by B1LYP/TZV(3df,2p) method on MP2 ropt structures. |
||||