| |
||||||||
| Table
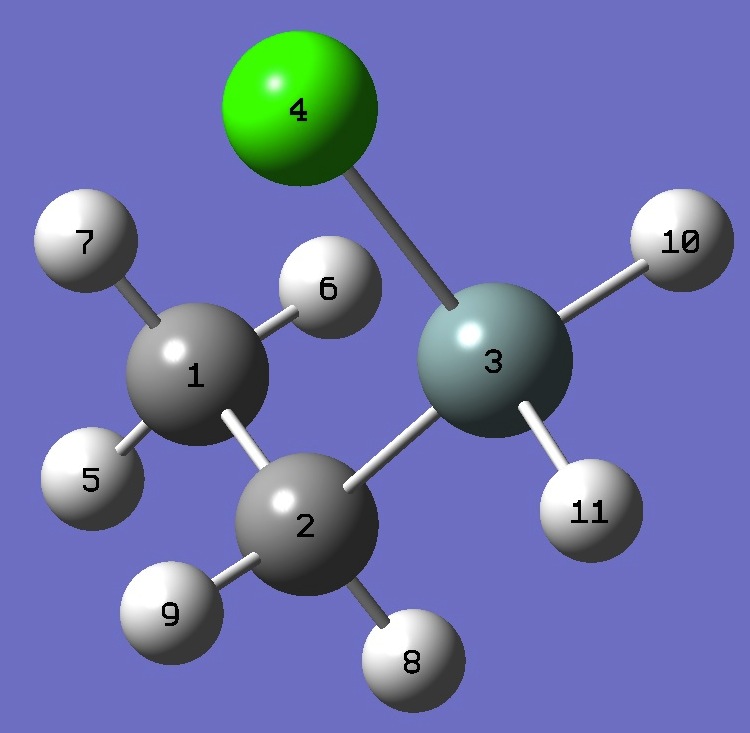
1. Chlorine nqcc's in gauche Ethylchlorosilane
(MHz). Calculation was made on experimental ro and MP2/6-311+G(d,p) optimized molecular structures. |
||||||||
| |
||||||||
| Calc /ro |
Calc /MP2 |
Expt. [1] * |
||||||
| Xaa (35Cl) | - |
10.25 |
- 9.01 |
- 8.989(82) |
||||
| Xbb | - 8.20 |
- 9.69 |
- 9.150(54) |
|||||
| Xcc | 18.45 |
18.70 |
18.139(54) |
|||||
| Xab | 27.67 |
28.32 |
||||||
| Xac | 4.20 |
4.55 |
||||||
| Xbc | - 4.72 |
- 5.05 |
||||||
| |
||||||||
| RMS |
0.93 (7.7 %) |
0.45 (3.7 %) |
||||||
| RSD | 0.49 (1.1 %) |
0.49 (1.1 %) | ||||||
| Xxx | 18.22 |
18.82 |
||||||
| Xyy | 19.41 |
19.65 |
||||||
| Xzz | - |
37.63 |
- |
38.47 |
||||
| ETA | 0.0315 |
0.0217 |
||||||
| Øz,SiCl | 0.55 |
0.73 |
||||||
| Xaa (37Cl) | - 8.59 |
- 7.63 |
- 7.08(60) | |||||
| Xbb | - 5.97 |
- 7.12 |
- 6.55(36) | |||||
| Xcc | 14.56 |
14.76 |
13.63(36) | |||||
| Xab | 21.80 |
22.33 |
||||||
| Xac | 3.30 |
3.58 |
||||||
| Xbc | - 3.63 |
- 3.89 |
||||||
| RMS |
1.08 (11.9 %) |
0.80 (8.8 %) |
||||||
| RSD |
0.44 (1.1 %) | 0.44 (1.1 %) | ||||||
| |
||||||||
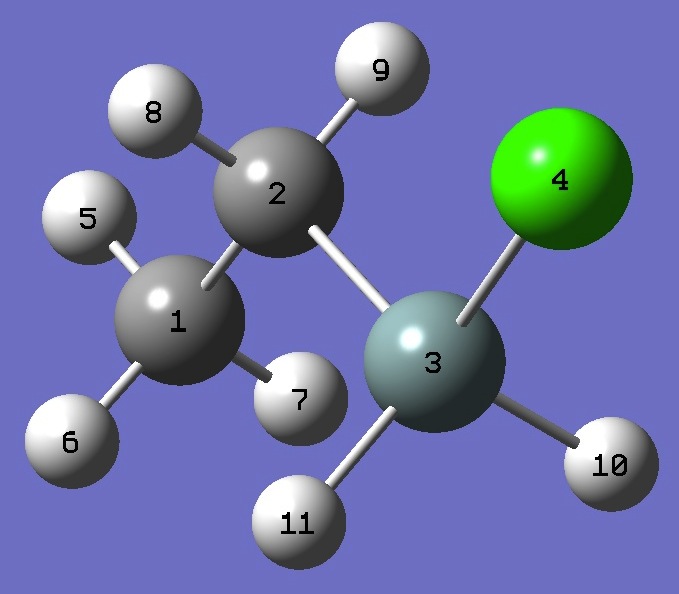
| Table 2. Chlorine nqcc's in trans Ethylchlorosilane
(MHz). Calculation was made on experimental ro and MP2/6-311+G(d,p) optimized molecular structures. |
||||||||
| |
||||||||
| Calc /ro |
Calc /MP2 |
Expt. [1] * |
||||||
| Xaa (35Cl) | - |
28.04 |
- |
27.69 |
- |
26.94(39) |
||
| Xbb | 8.63 |
8.00 |
7.9(17) |
|||||
| Xcc | 19.41 |
19.69 |
19.1(17) |
|||||
| |Xab| | 21.37 |
22.69 |
||||||
| |
||||||||
| RMS |
0.80 (4.4 %) |
0.56 (3.1 %) |
||||||
| RSD | 0.49 (1.1 %) |
0.49 (1.1 %) | ||||||
| Xxx | 18.45 |
19.02 |
||||||
| Xyy | 19.41 |
19.69 |
||||||
| Xzz | - |
37.86 |
- |
38.71 |
||||
| ETA | 0.0251 |
0.0173 |
||||||
| Øz,a | 24.68 |
25.91 |
||||||
| Øa,SiCl | 25.15 |
25.30 |
||||||
| Øz,SiCl | 0.47 |
0.60 |
||||||
| Xaa (37Cl) | - |
22.22 |
- |
21.95 |
- |
17.35(84) |
||
| Xbb | 6.93 |
6.43 |
11.6(54) |
|||||
| Xcc | 15.29 |
15.52 |
5.8(54) |
|||||
| |Xab| | 16.73 |
17.77 |
||||||
| RMS |
6.73 (58. %) |
6.76 (58. %) |
||||||
| RSD |
0.44 (1.1 %) | 0.44 (1.1 %) | ||||||
| |
||||
| Table 4. Ethylchlorosilane, 35Cl species. Rotational Constants (MHz). Calc = ro [1] and MP2/6-311+G(d,p) optimized molecular structures. | ||||
| Calc /ro | Calc /MP2 | Expt. [1] | ||
| gauche | A |
7189. |
7133. |
7191.8752(59) |
| B |
2587. |
2577. |
2585.1897(33) |
|
| C |
2085. |
2075. |
2083.6754(33) |
|
| trans | A |
14740. |
14710. |
14742.5(21) |
| B |
1876. |
1861. |
1876.7444(37) |
|
| C |
1734. |
1720. |
1733.8894(33) |
|