|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3CHClCN |
|
|
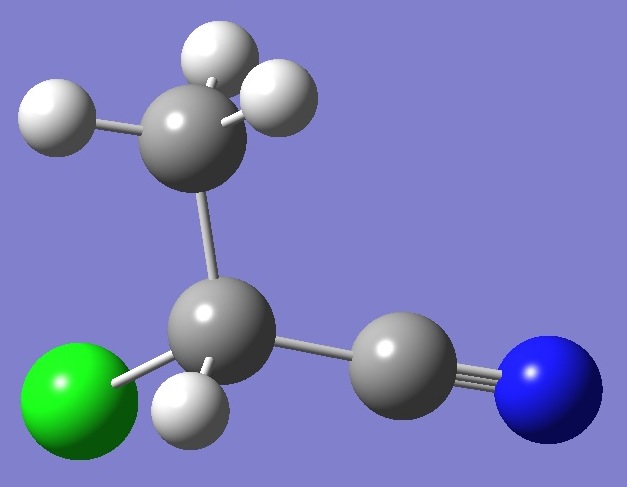
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine and Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in 2-Chloropropionitrile |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine and nitrogen nqcc tensors in 2-chloropropionitrile was made on a structure derived ab initio
as discussed below. Calculated chlorine nqcc's are compared with
the the experimental values in Tables 1 and 2. Nitrogen nqcc's
are shown in Tables 3 and 4. Structure parameters are given in
Table 5, atomic coordinates in Table 6, and rotational constants in
Table 7. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 4, subscripts a,b,c refer to the principal axes of the inertia
tensor, subscripts x,y,z to the principal axes of the nqcc tensor. Ø (degrees)
is the angle between its subscripted parameters. ETA = (Xxx
- Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental nqcc's (percentage of
the average experimental nqcc). RSD is the residual standard deviation
of calibration of the model for calculation of
the nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. 35Cl nqcc's in CH3CHClCN (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
35Cl |
Xaa |
- |
31.14 |
- |
28.24(51) |
|
|
|
Xbb |
|
1.78 |
|
0.97 ** |
|
|
|
Xcc |
|
29.36 |
|
27.27 ** |
|
|
|
Xab* |
- |
50.76 |
|
|
|
|
|
Xac* |
- |
26.02 |
|
|
|
|
|
Xbc* |
- |
19.25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
2.12 (11.2 %) |
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
38.57 |
|
|
|
|
|
Xyy |
|
39.24 |
|
|
|
|
|
Xzz |
- |
77.82 |
|
|
|
|
|
ETA |
|
0.009 |
|
|
|
|
|
Øz,CCl |
|
1.54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Algebraic signs of the the off-diagonal nqcc's correspond to the molecular a,b,c coordinates given below in Table 6. |
|
|
** Derived here from the experimental Xaa and Xbb - Xcc = - 26.30(63) MHz [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. 37Cl nqcc's in CH3CHClCN (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
37Cl |
Xaa |
- |
26.09 |
- |
23.41(93) |
|
|
|
Xbb |
|
2.87 |
|
0.60 ** |
|
|
|
Xcc |
|
23.22 |
|
22.80 ** |
|
|
|
Xab* |
- |
39.53 |
|
|
|
|
|
Xac* |
- |
20.69 |
|
|
|
|
|
Xbc* |
- |
14.71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
2.04 (13.1 %) |
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
30.40 |
|
|
|
|
|
Xyy |
|
30.93 |
|
|
|
|
|
Xzz |
- |
61.33 |
|
|
|
|
|
ETA |
|
0.009 |
|
|
|
|
|
Øz,CCl |
|
1.54 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
** Derived here from the experimental Xaa and Xbb - Xcc = - 22.20(86) MHz [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 3. 14N nqcc's
in CH3CH35ClCN (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
2.805 |
|
|
|
|
|
Xbb |
|
1.056 |
|
|
|
|
|
Xcc |
|
1.749 |
|
|
|
|
|
Xab* |
|
2.447 |
|
|
|
|
|
Xac* |
|
1.352 |
|
|
|
|
|
Xbc* |
- |
0.486 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.996 |
|
|
|
|
|
Xyy |
|
2.332 |
|
|
|
|
|
Xzz |
- |
4.327 |
|
|
|
|
|
ETA |
|
0.078 |
|
|
|
|
|
Øz,CN |
|
0.16 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Algebraic signs of the the off-diagonal nqcc's correspond to the molecular a,b,c coordinates given below in Table 6. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 4. 14N nqcc's
in CH3CH37ClCN (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
2.708 |
|
|
|
|
|
Xbb |
|
0.961 |
|
|
|
|
|
Xcc |
|
1.747 |
|
|
|
|
|
Xab* |
|
2.519 |
|
|
|
|
|
Xac* |
|
1.345 |
|
|
|
|
|
Xbc* |
- |
0.513 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular Structure |
|
|
|
|
|
|
|
|
|
|
|
|
The molecular structure was optimized
at the MP2/6-311G(d,p) level of theory. The optimized C-C,
C-C(N), and CN bond lengths were corrected using equations obtained
from linear regression analyses of the data given in Tables VIII and IX
of Ref. [2]. For the CCl bond, the structure was optimized
at the MP2/6-311+G(2d,p) level and corrected by linear regression
analysis of the data given in Table 4 of Ref. [3]. The CH bond
lengths were corrected using r = 1.001 ropt, where ropt
is obtained by MP2/6-31G(d,p) optimization [4]. Interatomic
angles used in the calculation are those given by MP2/6-311+G(2d,p)
optimization. |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| Table 5.
2-Chloropropionitrile. Heavy atom structure parameters
(Å and degrees). The complete structure is given here in Z-Matrix format. |
| |
|
|
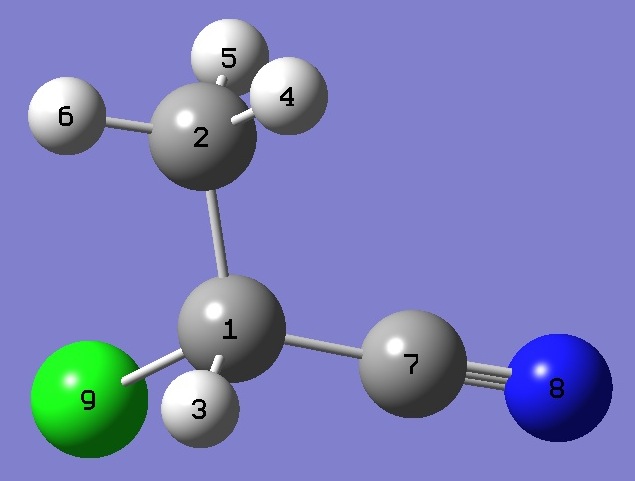 |
NC(7) |
1.1566 |
| C(7)C(1) |
1.4601 |
| C(1)Cl |
1.7874 |
| C(1)C(2) |
1.5162 |
| NC(7)C(1) |
178.04 |
| C(7)C(1)C(2) |
111.79 |
| C(2)C(1)Cl |
110.56 |
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 6. 2-Chloropropionitrile. Atomic coordinates. Normal species. |
| |
|
|
|
|
|
|
|
|
|
|
a (Å) |
|
b (Å) |
|
c (Å) |
|
|
|
|
|
|
|
|
|
C(1) |
|
0.0251 |
|
0.4343 |
|
0.4431 |
|
C(2) |
- |
0.0889 |
|
1.8048 |
- |
0.1953 |
|
H(3) |
- |
0.0481 |
|
0.5009 |
|
1.5286 |
|
H(4) |
|
0.7343 |
|
2.4332 |
|
0.1447 |
|
H(5) |
- |
0.0464 |
|
1.7214 |
- |
1.2793 |
|
H(6) |
- |
1.0329 |
|
2.2644 |
|
0.0907 |
|
C(7) |
|
1.2860 |
- |
0.2188 |
|
0.1031 |
|
N(8) |
|
2.3033 |
- |
0.7016 |
- |
0.1608 |
|
Cl(9) |
- |
1.3304 |
- |
0.6118 |
- |
0.0700 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
| Table 7. 2-Chloropropionitrile. Rotational constants (MHz). Normal species. |
| |
|
|
|
|
|
Calc. ropt |
Expt. [1] |
|
|
|
|
|
A |
6031.7 |
5973.3196(104) |
|
B |
3058.1 |
3049.4864(79) |
|
C |
2159.5 |
2147.1990(66) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] T.Ogata, N.Yamashita, and S.Takata, J.Mol.Struct. 412,39(1997). |
|
|
[2] J.Demaison, J.Cosléou, R.Bocquet, and A.G.Lesarri, J.Mol.Spectrosc. 167,400(1994). |
|
|
[3] I.Merke, L.Poteau, G.Wlodarczak, A.Bouddou, and J.Demaison, J.Mol.Spectrosc. 177,232(1996). |
|
|
[4] J.Demaison and G.Wlodarczak, Structural Chem. 5,57(1994). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
g-3-Chloropropionitrile |
t-3-Chloropropionitrile |
|
|
|
|
Ethyl Cyanide |
Chloroacetonitrile |
|
|
|
|
Ethyl Chloride
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3CHClCN.html |
|
|
|
|
|
|
Last
Modified 1 July 2006 |
|
|
|
|
|
|
|
|
|
|

