|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
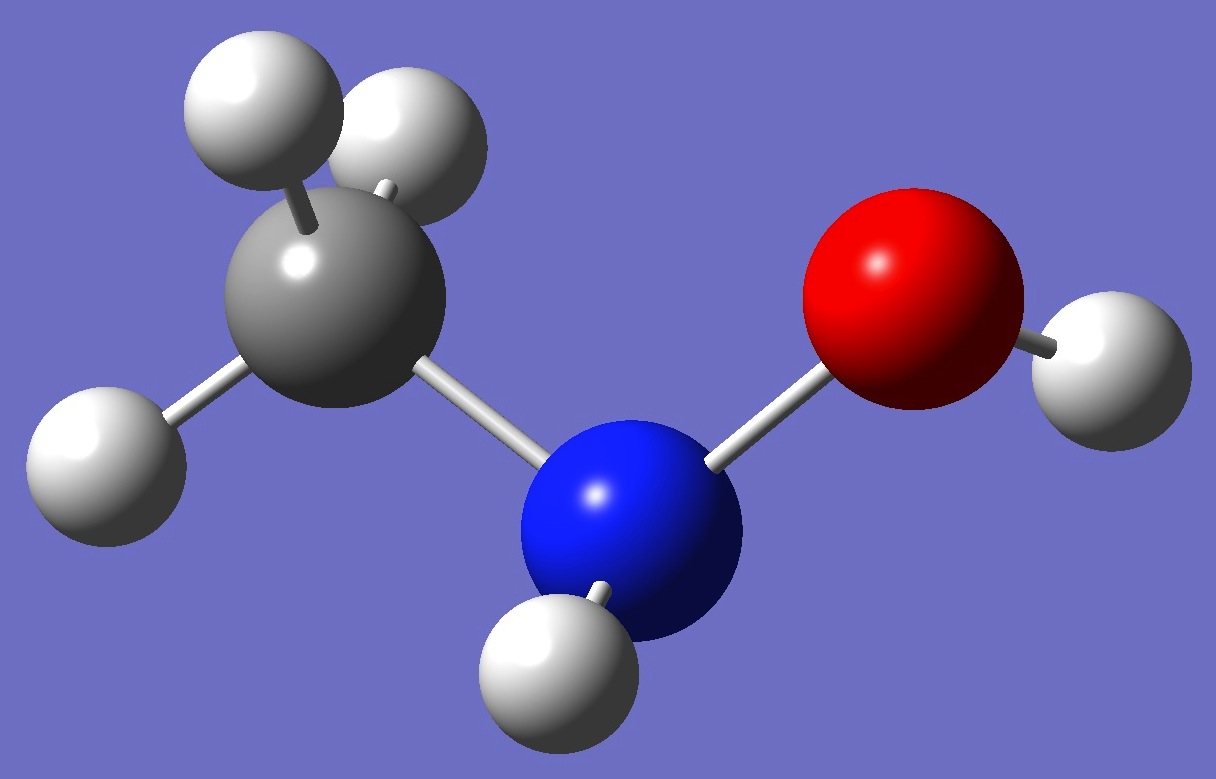
(CH3)N(H)OH
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in N-Methylhydroxylamine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular conformation and 14N
nuclear quadrupole coupling constants in N-methylhydroxylamine were
first investigated by Sung and Harmony [1], and subsequently revisited by Kolesniková et al. [2].
|
|
|
Calculation of the 14N
nuclear quadrupole coupling constants was made here on a partial ro molecular
structure reported in Ref. [1], and on an ropt structure derived here by MP2/aug-cc-pVTZ optimization.
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculated
and experimental
nqcc's are compared in Tables 1 and 2. Structure parameters are
given in Table 3, rotational constants and dipole moments in
Table 4.
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. RMS is the root
mean square difference between calculated and experimental diagonal
nqcc's. RSD is the calibration residual standard
deviation of the B3PW91/6-311+G(df,pd) model for calculation of the nitrogen efg's/nqcc's.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N nqcc's in N-Methylhydoxylamine (MHz). Calculation was made
on the ro and ropt structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /ro
|
|
Calc /ropt
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
4.345
|
|
4.279
|
|
4.35(1)
|
|
|
Xbb
|
|
0.730
|
|
0.681
|
|
0.67(1)
|
|
|
Xcc
|
-
|
5.075
|
-
|
4.960
|
-
|
5.02(2)
|
|
|
Xab
|
|
2.782
|
|
2.753
|
|
0.2(1)
|
|
|
Xac |
-
|
0.703
|
-
|
0.678
|
-
|
0.4(4)
|
|
|
Xbc |
|
2.844
|
|
2.909
|
-
|
3.03(2)
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.047 (1.4 %)
|
|
0.054 (1.6 %)
|
|
|
|
|
RSD
|
|
0.030 (1.3 %)
|
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx
|
|
0.608
|
|
0.642
|
|
|
|
|
Xyy
|
|
5.911
|
|
5.834
|
|
|
|
|
Xzz
|
-
|
6.519
|
-
|
6.476
|
|
|
|
|
ETA
|
|
0.813
|
|
0.802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N nqcc's in N-Methylhydoxylamine (MHz). Calculation was made
on the ro and ropt structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /ro
|
|
Calc /ropt
|
|
Expt [2]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
4.345
|
|
4.279
|
|
4.3434(96)
|
|
|
Xbb
|
|
0.730
|
|
0.681
|
|
0.7126(72)
|
|
|
Xcc
|
-
|
5.075
|
-
|
4.960
|
-
|
5.0560(64)
|
|
|
Xab
|
|
2.782
|
|
2.753
|
|
|
|
|
Xac |
-
|
0.703
|
-
|
0.678
|
|
|
|
|
Xbc |
|
2.844
|
|
2.909
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.015 (0.44 %)
|
|
0.069 (2.0 %)
|
|
|
|
|
RSD
|
|
0.030 (1.3 %)
|
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3. N-Methylhydoxylamine: Structure parameters, ro and ropt (Ĺ and degrees). For the ro structure, the methyl hydrogen geometry is taken from the ropt structure.
|
|
|
|
|
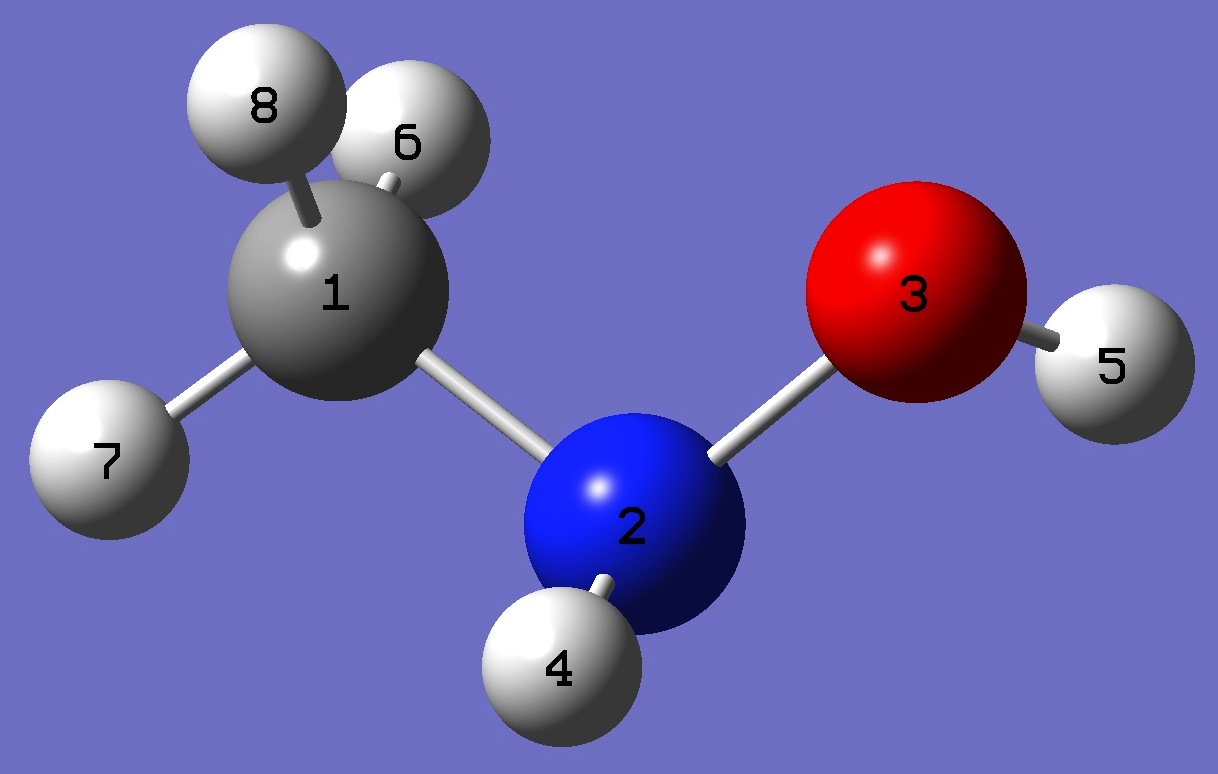
|
C
N,1,R1
O,2,R2,1,A1
H,2,R3,1,A2,3,D1,0
H,3,R4,2,A3,1,D2,0
H,1,R5,2,A4,3,D3,0
H,1,R6,2,A5,3,D4,0
H,1,R7,2,A6,3,D5,0
|
|
|
| ro (with uncertainties) |
ropt
|
|
|
|
|
R1=1.460(8)
R2=1.461(10)
R3=1.007(10)
R4=0.962(10)
R5=1.08692431
R6=1.08980259
R7=1.0925917
A1=106.4(5)
A2=108.3(10)
A3=100.8(15)
A4=109.0247985
A5=107.56296921
A6=112.7000713
D1=109.48667641
D2=-126.4(15)
D3=71.52217516
D4=-170.60658985
D5=-49.74430872
|
R1=1.45636916
R2=1.4454866
R3=1.01566076
R4=0.96331518
R5=1.08692431
R6=1.08980259
R7=1.0925917
A1=106.41457981
A2=107.93007114
A3=102.05634129
A4=109.0247985
A5=107.56296921
A6=112.7000713
D1=109.48667641
D2=-125.88393826
D3=71.52217516
D4=-170.60658985
D5=-49.74430872
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4. N--Methylhydoxylamine: Rotational Constants (MHz) and Dipole Moments * (D). |
|
|
|
|
|
|
Calc /ro
|
Calc /ropt
|
Expt [1]
| Expt [2] |
|
|
|
|
|
A
|
38832.
|
39129.
|
38930.771(5)
| 38930.75277(57)
|
B
|
9929.
|
10033.
|
9939.607(2)
| 9939.618023(99)
|
C
|
8677.
|
8778.
|
8690.716(1)
| 8690.696797(91)
|
|
|
|µa|
|
0.694
|
0.639
|
0.611(8)
|
|
| |µb| |
0.480
|
0.438
|
0.366(37)
|
|
| |µc| |
0.054
|
0.061
|
------
|
|
|
|
|
|
|
* Calculated on the indicated structure by B3PW91/6-311+G(df,pd) method.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] E.-M.Sung and M.D.Harmony, J.Mol.Spectrosc. 74,228(1979).
|
|
|
[2] L.Kolesniková, E.R.Alonso, S.Mata, and J.L.Alonso, J.Mol.Spectrosc. 335,54(2017).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3NHOH.html |
|
|
|
|
|
|
Last
Modified 17 March 2017 |
|
|
|
|
|
|
|
|
|
|

