|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3NO2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Nitromethane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen nqcc's in nitromethane, as well as a substitution structure, were determined by Cox and Waring [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the nqcc's was made here on this rs molecular structure. Calculated and experimental nqcc's are compared in
Table 1. Structure parameters are given in Table 2.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean
square difference between calculated and experimental diagonal nqcc's
(percentage of the average of the magnitudes of the experimental
nqcc's). RSD is the calibration residual standard deviation of
the (1) B3PW91/6-311+G(df,pd) and (2) B3PW91/6-311+G(d,p) models for calculation of nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc's in Nitromethane (MHz). Calculation was made on the rs structure with both (1)
B3PW91/6-311+G(df,pd) and (2) B3PW91/6-311+G(d,p) models. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. (1)
|
|
Calc. (2) |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
1.236 |
-
|
1.233 |
-
|
1.185(7) |
|
|
Xbb |
|
0.222 |
|
0.373 |
|
0.305(12) |
|
|
Xcc |
|
1.014 |
|
0.860 |
|
0.880(12) |
|
|
|Xab| |
|
0.010 |
|
0.011 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.096 (12. %) |
|
0.050 (6.3 %) |
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.086 (3.8 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.222 |
|
0.373 |
|
|
|
|
Xyy |
|
1.014 |
|
0.860 |
|
|
|
|
Xzz |
- |
1.236 |
- |
1.233 |
|
|
|
|
ETA |
|
0.640 |
|
0.395 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Table 2. Nitromethane. Z-Matix structure parameters derived from rs coordinates [1] (Å and degrees).
|
| |
|
|
|
|
|
|
|
|
|
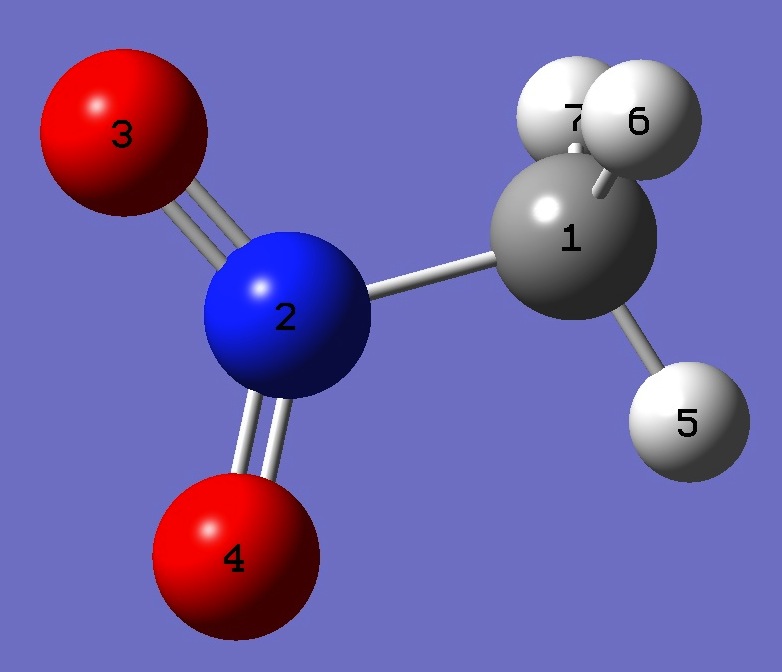
|
C
N 1 B1
O 2 B2 1 A1
O 2 B3 1 A2 3 D1
H 1 B4 2 A3 3 D2
H 1 B5 2 A4 3 D3
H 1 B6 2 A5 3 D4
|
|
|
|
B1 1.49100011
B2 1.22429317
B3 1.22429317
B4 1.08871575
B5 1.08873757
B6 1.08873757
A1 117.26201012
A2 117.26201012
A3 107.34648984
A4 107.34613112
A5 107.34613112
D1 180.00000000
D2 180.00000000
D3 60.00072777
D4 -60.00072777
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] A.P.Cox and S.Waring, J.Chem.Soc. Faraday Trans. II 68,1060(1972).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitroethylene
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3NO2.html |
|
|
|
|
|
|
Last
Modified 28 Aug 2015 |
|
|
|
|
|
|
|
|
|
|

