|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
C6H11Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in equatorial Cyclohexyl Chloride |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine nqcc's
in equatorial cyclohexyl chloride was made on a molecular structure derived ab
initio,
as described below. These are compared in Table 1 with the
experimental nqcc's of Caminati, et al. [1]. Structure parameters
are given in Table 2, rotational constants in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, RMS is the root mean square difference
between calculated and experimental diagonal nqcc's (percentage of the
average of the magnitudes of the experimental nqcc's). RSD is the
calibration residual standard deviation for the B1LYP/TZV(3df,2p) model
for calculation of the chlorine nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
Subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. The nqcc y-axis is chosen coincident with the
inertia b-axis, these are perpendicular to the molecular symmetry plane.
Ø (degrees) is the angle between its subscripted
parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Chlorine
nqcc's in cyclohexyl chloride, equatorial (MHz). |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
35Cl |
Xaa |
- |
62.26 |
- |
61.4(2) |
|
|
|
Xbb |
|
34.42 |
|
30.3(12) |
|
|
|
Xcc |
|
27.84 |
|
31.1 |
|
|
|
|Xac| |
|
24.50 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
3.1 (7.5 %) |
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
34.07 |
|
|
|
|
|
Xyy |
|
34.42 |
|
|
|
|
|
Xzz |
- |
68.49 |
|
|
|
|
|
ETA |
|
0.005 |
|
|
|
|
|
Øz,a |
|
14.27 |
|
|
|
|
|
Øa,CCl |
|
13.67 |
|
|
|
|
|
Øz,CCl |
|
0.60 |
|
|
|
|
|
|
|
|
|
|
|
|
37Cl |
Xaa |
- |
49.09 |
- |
43.8(8) |
|
|
|
Xbb |
|
27.12 |
|
27.5(19) |
|
|
|
Xcc |
|
21.97 |
|
16.3 |
|
|
|
|Xac| |
|
19.26 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
4.5 (15 %) |
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Molecular Structure |
|
|
|
|
|
|
|
|
|
|
|
|
The molecular structure was optimized
at the MP2/6-311G(d,p) level of theory assuming Cs symmetry.
The optimized CC bond lengths were then corrected using the equation
obtained from linear regression analysis of the data given in Table
IX of Ref. [2]. For CCl, the structure was optimized at
the MP2/6-311+G(2d,p) level and the bond length corrected by linear regression analysis
of the data given in Table 4 of Ref. [3]. The CH bond lengths were
corrected using r = 1.001 ropt, where ropt is obtained
by MP2/6-31G(d,p) optimization [4]. Interatomic angles used in the
calculation are those given by MP2/6-311+G(2d,p) optimization. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
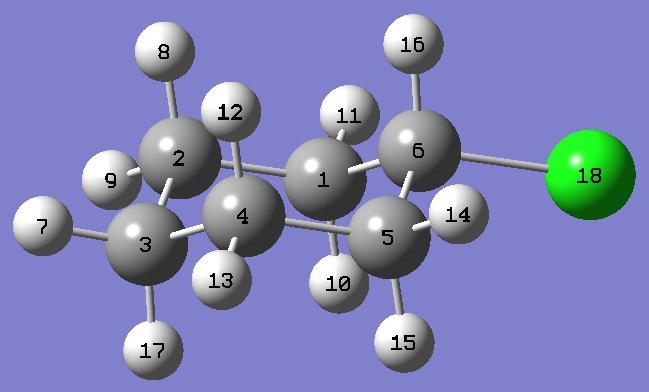
| Table 2. Cyclohexyl Chloride, equatorial. Heavy atom structure parameters, ropt (Å and degrees). The complete structure is given here in Z-Matrix format. |
| |
|
|
| Atomic numbering |
C(6)Cl |
1.7944 |
|
C(6)C(5) |
1.5151 |
|
C(5)C(4) |
1.5254 |
| C(4)C(3) |
1.5232 |
| CCC,Cl * |
127.70 |
| C(1)C(6)C(5) |
111.76 |
| C(6)C(5)C(4) |
109.92 |
| C(5)C(4)C(3) |
111.05 |
| C(4)C(3)C(2) |
110.69 |
|
|
|
* Angle that C(6)Cl makes with the C(1)C(6)C(5) plane. |
| On the rs structure of Ref. [1] and the ro structure of Ref. [5], the CCl bond lengths are respectively 1.804(4) and 1.7997(2) Å. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3. Cyclohexyl Chloride, equatorial. Rotational Constants (MHz). 35Cl
species. |
| |
|
|
|
|
|
Calc. ropt |
Expt. [1] |
|
|
|
|
|
A |
4352.3 |
4292.09(9) |
|
B |
1411.0 |
1396.982(5) |
|
C |
1139.8 |
1127.346(4) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] W.Caminati, F.Scappini, and D.Damiani, J.Mol.Spectrosc. 108, 287(1984). |
|
|
[2] J.Demaison, J.Cosléou, R.Bocquet,
and A.G.Lesarri, J.Mol.Spectrosc. 167,400(1994). |
|
|
[3] I.Merke, L.Poteau, G.Wlodarczak, A.Bouddou,
and J.Demaison, J.Mol.Spectrosc. 177,232(1996). |
|
|
[4] J.Demaison and G.Wlodarczak, Structural
Chem. 5,57(1994). |
|
|
[5] E.Białkowska-Jaworska, M.Jaworski, and Z.Kisiel, J.Mol.Struct. 350,247(1995). |
|
|
|
|
|
|
|
|
|
|
|
|
D.Damiani and L.Ferretti, Chem.Phys.Lett. 21,592(1973). Xaa, Xbb, and Xcc = -61.4(2), 30.5(7), and 30.9(9) MHz, respectively. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cyclohexyl Chloride, axial |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Clcychex_eq.html |
|
|
|
|
|
|
Last
Modified 4 May 2006 |
|
|
|
|
|
|
|
|
|
|

