|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
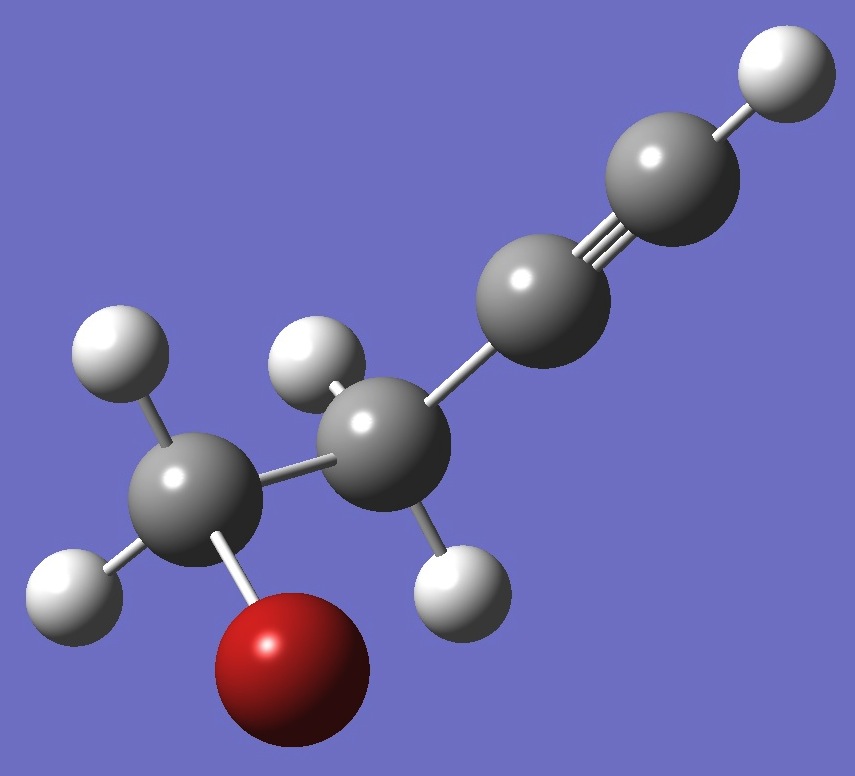
HCC-CH2CH2Br
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bromine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in gauche Bromobutyne |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Complete nqcc tensors for both 79Br and 81Br in gauche bromobutyne were determined by Keske et al. [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the nqcc tensors was made here on molecular structures derived by MP2/aug-cc-pVTZ optimization (ropt), and on this same structure but with corrected CBr, C-C, and triple CC bond lengths (~ re, see here). These calculated nqcc's are compared with the
experimental values in Tables 1 and 2. Structure parameters are
given in Table 3, rotational constants in Table 4. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the principal axes of the inertia
tensor; x,y,z to the principal axes of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscripted
parameters.
|
|
|
RMS is the root mean square difference
between calculated and experimental diagonal nqcc's (percentage of the
average of the magnitudes of the experimental nqcc's). RSD is the
calibration residual standard deviation for the B1LYP/TZV(3df,3p) model
for calculation of the efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 79Br nqcc's in gauche HCC-CH2CH2Br (MHz). Calculation was made on (1) the ropt structure, and (2) the ~ re structure. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2) |
|
Expt. [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
54.20
|
|
59.04
|
|
64.3368(61) |
|
|
Xbb |
|
162.17 |
|
158.69 |
|
153.169(6) *
|
|
|
Xcc |
- |
216.37 |
- |
217.73 |
- |
217.506(6) *
|
|
|
Xab
|
|
390.80 |
|
392.76 |
|
390.214(65)
|
|
|
Xac ** |
|
138.08
|
|
138.51
|
-
|
138.87(28)
|
|
|
Xbc ** |
|
164.64
|
|
163.08
|
-
|
163.437(77)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
7.85 (5.42 %)
|
|
4.42 (3.04 %) |
|
|
|
|
RSD |
|
1.58 (0.39 %) |
|
1.58 (0.39 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
- |
287.60 |
- |
288.23 |
- |
286.54 |
|
|
Xyy |
- |
274.39 |
- |
275.14 |
- |
274.02 |
|
|
Xzz |
|
561.99 |
|
563.37 |
|
560.57 |
|
|
ETA |
-
|
0.0235 |
-
|
0.0232 |
|
|
|
|
Øz,CBr |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa = 64.3368(61) and (Xbb - Xcc) = 370.675(11) MHz using Kisiel's QDIAG.f. See http://info.ifpan.edu.pl/~kisiel/prospe.htm
|
|
|
** Difference in algebraic sign between calc and expt is not significant. In each case, the algebraic sign of the product XabXacXbc is positive. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 81Br nqcc's in gauche HCC-CH2CH2Br (MHz). Calculation was made on (1) the ropt structure, and (2) the ~ re structure. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2) |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
46.76
|
|
50.80
|
|
55.0768(82) |
|
|
Xbb |
|
134.14 |
|
131.24 |
|
126.705(7)
|
|
|
Xcc |
- |
180.90 |
- |
182.04 |
- |
181.782(7)
|
|
|
Xab
|
|
326.76 |
|
328.38 |
|
326.314(53) |
|
|
Xac ** |
|
115.52
|
|
115.88
|
-
|
115.93(20)
|
|
|
Xbc ** |
|
137.13
|
|
135.84
|
-
|
135.496(75)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
6.46 (5.33 %) |
|
3.60 (2.97 %) |
|
|
|
|
RSD |
|
1.38 (0.40 %) |
|
1.38 (0.40 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa = 55.0768(82) and (Xbb - Xcc) = 308.487(11) MHz using Kisiel's QDIAG.f. See http://info.ifpan.edu.pl/~kisiel/prospe.htm
|
|
|
** Difference in algebraic sign between calc and expt is not significant. In each case, the algebraic sign of the product XabXacXbc is positive.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
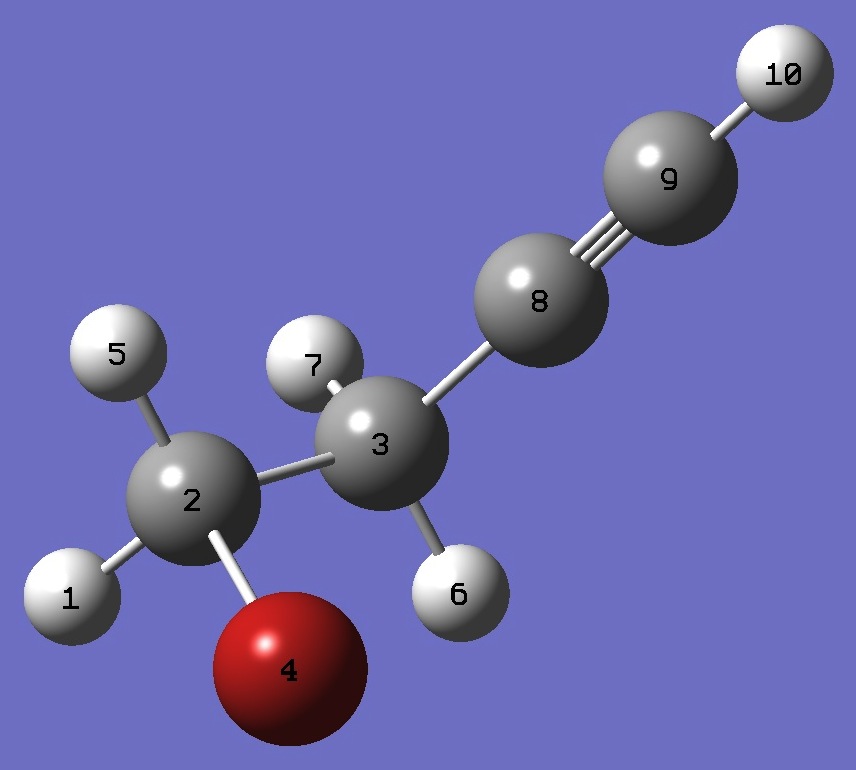
| Table 3. gauche HCC-CH2CH2Br. Molecular structure parameters, ropt = MP2/aug-cc-pVTZ, approximate equilibrium bond lengths (~ re) are given in parentheses (Å and degrees). |
| |
|
|
|
|
H
C,1,B1
C,2,B2,1,A1
Br,2,B3,1,A2,3,D1,0
H,2,B4,1,A3,3,D2,0
H,3,B5,2,A4,1,D3,0
H,3,B6,2,A5,1,D4,0
C,3,B7,2,A6,1,D5,0
C,8,B8,3,A7,1,D6,0
H,9,B9,8,A8,1,D7,0
|
|
|
|
|
|
|
|
B1=1.08594755
B2=1.52069954 (1.5141)
B3=1.92991679 (1.9380)
B4=1.0858269
B5=1.09101138
B6=1.09360482
B7=1.45734087 (1.4517)
B8=1.21537495 (1.2044)
B9=1.06191057
A1=110.97657022
A2=106.32823843
A3=110.04186464
A4=109.46283598
A5=107.96505092
A6=112.6170906
A7=178.68262553
A8=179.34572632
D1=-121.21301621
D2=123.88999685
D3=-61.19766639
D4=55.40179118
D5=176.2261373
D6=-39.13791564
D7=100.27213826
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4. gauche HCC-CH2CH2Br. Rotational constants (MHz). 79Br species. |
| |
|
|
|
|
| |
|
ropt |
~ re |
Expt. [1] |
|
|
|
|
|
|
A |
6818.
|
6840.
|
6880.1243(24)
|
|
B |
1694.
|
1699.
|
1651.54449(40)
|
|
C |
1427.
|
1431.
|
1398.48178(41)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] J.C.Keske,
F.S.Rees, R.D.Suenram, and B.H.Pate, PCCP 5,1599(2003). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
trans-HCC-CH2CH2Br |
HCC-CH2Br |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Bromine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCCCH2CH2Br_gau.html |
|
|
|
|
|
|
Last
Modified 26 Dec 2016 |
|
|
|
|
|
|
|
|
|
|

