|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HOCH2CN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Hydroxyacetonitrile
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the nitrogen
nqcc tensors in the gauche and trans conformers of hydroxyacetonitrile was made on molecular structures given by MP2/aug-cc-pVTZ optimization with approximate re CC and CN bond lengths. These calculated nqcc's are compared with the experimental values for the gauche conformer [1] in Table 1, and are given for the trans
conformer in Table 2.
Structure parameters are given in Table 3 in Z-matrix format,
rotational constants and dipole moments in Table 4, quartic centrifugal
distortions constants in Table 5.
|
|
|
|
|
|
|
|
|
|
|
|
|
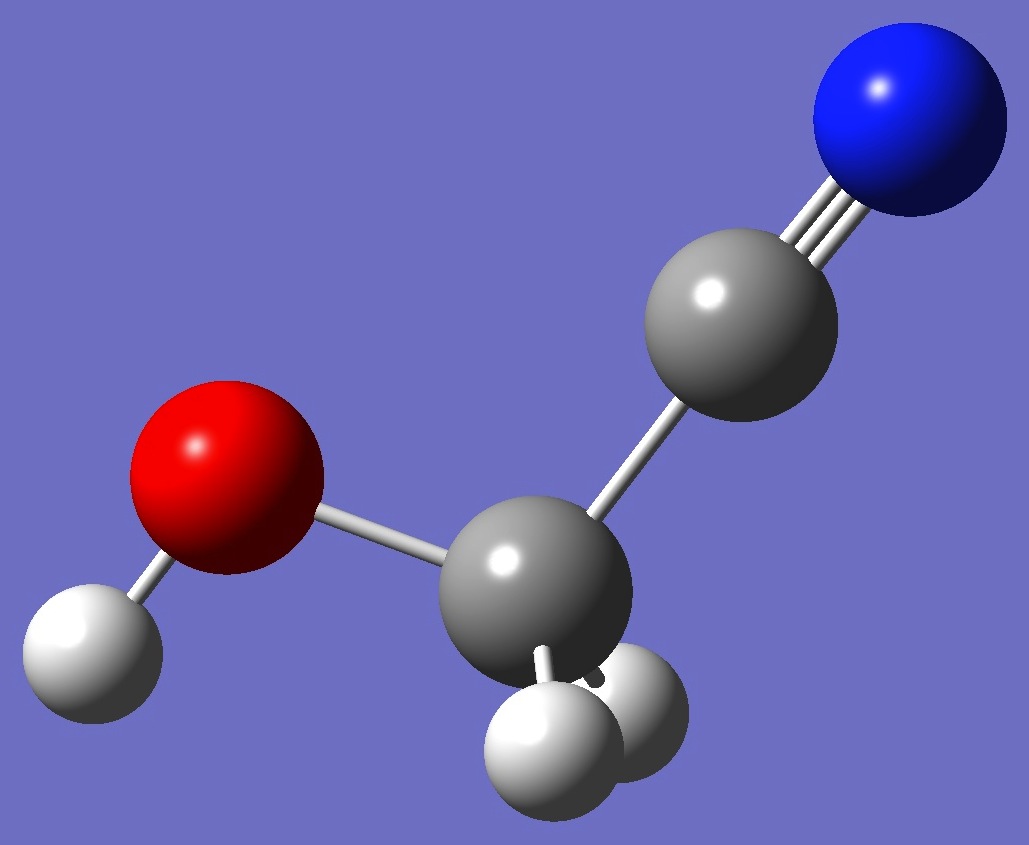
trans Cs
|
|
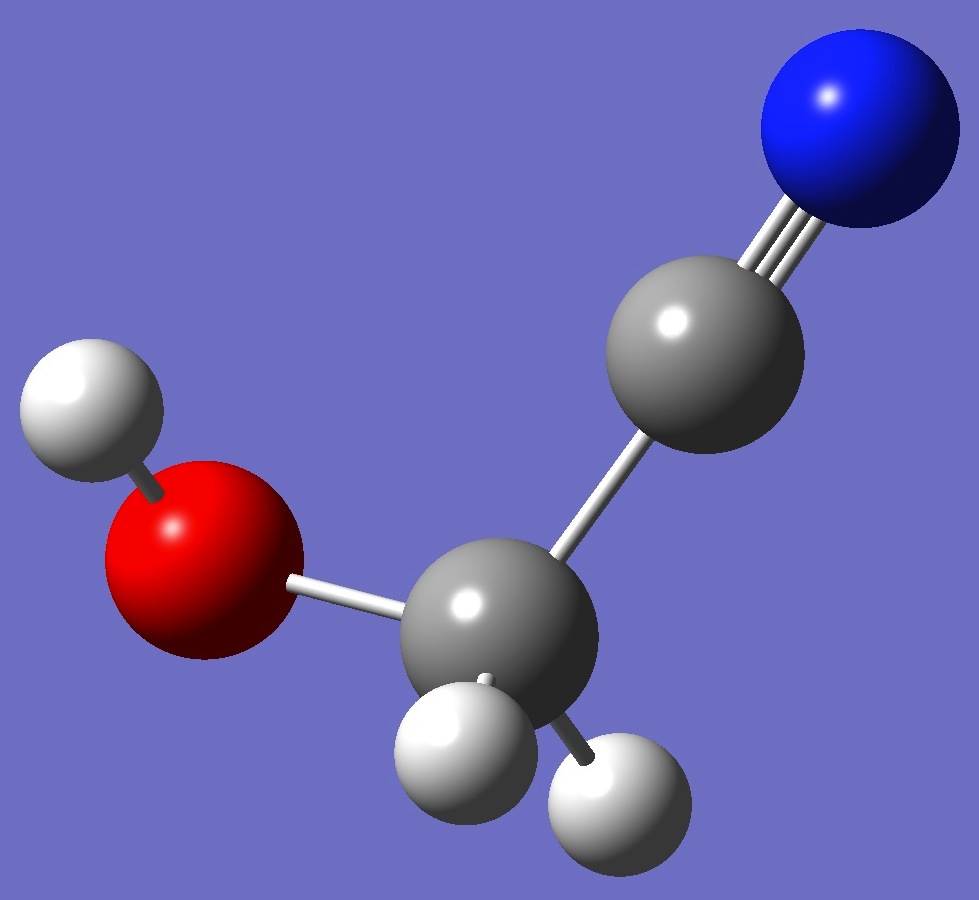
gauche C1
|
|
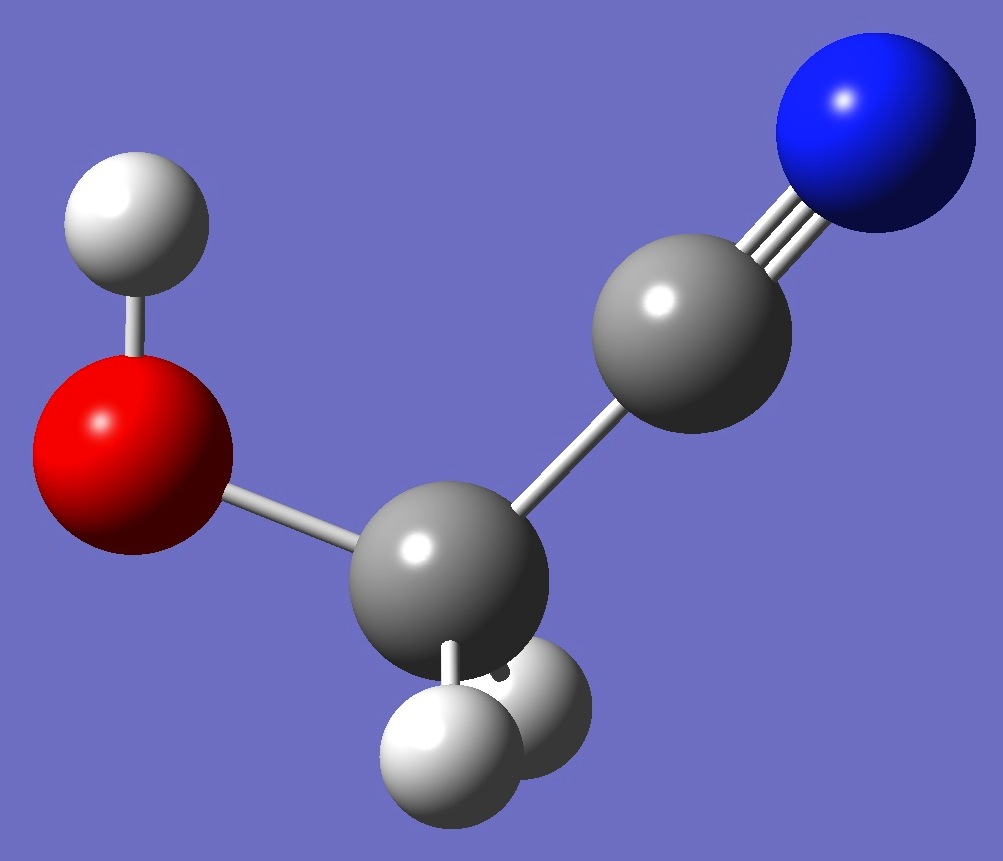
cis Cs |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Local Minimum
|
|
Global Minimum
|
|
TS Saddle Point
|
|
|
|
|
|
|
|
|
|
|
|
|
At the B3LYP/cc-pVTZ and MP2/cc-pVTZ levels of theory respectively, trans hydroxyacetonitrile is higher in energy than gauche by 7.7/7.1 kJ/mol, while cis is higher than gauche by 5.3/5.9 kJ/mol. The cis conformer - with one imaginary frequency - is a saddle point on the PE surface, presumable connecting equivalent mirror image gauche conformers.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, RMS is the root mean square difference between calculated and experimental diagonal nqcc's. RSD is the
calibration residual standard deviation of the B3PW91/6-311+G(df,pd) model
for calculation of the nitrogen nqcc's. |
|
|
Subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscripted
parameters.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc's in gauche HOCH2CN (MHz). Calculation was made on a molecular structure given by MP2/aug-cc-pVTZ optimization with approximate re CC and CN bond lengths.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
3.561 |
-
|
3.63
|
|
|
|
Xbb |
|
1.697 |
|
1.64
|
|
|
|
Xcc |
|
1.864
|
|
1.99
|
|
|
|
Xab |
|
2.248
|
|
|
|
|
|
Xac |
|
0.011
|
|
|
|
|
|
Xbc |
-
|
0.055
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.097 (4.0 %)
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.531 |
|
|
|
|
|
Xyy |
|
1.861 |
|
|
|
|
|
Xzz |
- |
4.392 |
|
|
|
|
|
ETA |
|
0.152 |
|
|
|
|
|
Øz,CN |
|
0.31
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen
nqcc's in trans HOCH2CN (MHz). Calculation was made on a molecular structure given by MP2/aug-cc-pVTZ optimization with approximate re CC and CN bond lengths.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
3.564 |
|
|
|
|
|
Xbb |
|
1.826 |
|
|
|
|
|
Xcc |
|
1.828
|
|
|
|
|
|
Xab |
|
2.122
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.552 |
|
|
|
|
|
Xyy |
|
1.828 |
|
|
|
|
|
Xzz |
- |
4.380 |
|
|
|
|
|
ETA |
-
|
0.165 |
|
|
|
|
|
Øz,a |
|
18.88
|
|
|
|
|
|
Øa,CN |
|
18.54
|
|
|
|
|
|
Øz,CN |
|
0.34
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
Table 3. Hydroxyacetonitrile. MP2/aug-cc-pVTZ optimized molecular structure parameters (Å
and degrees). Approximate re bond lengths for CC and CN are given in parentheses.
|
| |
|
|

|
N
C,1,B1
C,2,B2,1,A1
O,3,B3,2,A2,1,D1,0
H,4,B4,3,A3,2,D2,0
H,3,B5,2,A4,1,D3,0
H,3,B6,2,A5,1,D4,0
|

|
|
|
|
trans
|
|
gauche
|
|
|
|
B1=1.16963183 (1.1552)
B2=1.46354819 (1.4640)
B3=1.41816573
B4=0.96343489
B5=1.09243859
B6=1.09243859
A1=178.13717936
A2=107.94457755
A3=107.62920186
A4=108.15046671
A5=108.15046671
D1=180.
D2=180.
D3=-58.75396373
D4=58.75396373
|
|
B1=1.17062545 (1.1559)
B2=1.47132978 (1.4715)
B3=1.41360373
B4=0.96360414
B5=1.09189219
B6=1.0873618
A1=178.20693054
A2=112.48707348
A3=108.43138072
A4=108.41569433
A5=108.85726323
D1=-52.38789233
D2=60.63215579
D3=72.35692477
D4=-170.15610614
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 4. Hydroxyacetonitrile. Rotational Constants (MHz) and Dipole Moments * (D).
|
|
|
|
|
|
|
|
|
trans
|
|
gauche
|
|
|
|
Calc
|
Expt
|
Calc
|
Expt [1]
|
|
|
|
|
|
|
|
A
|
35375
|
|
33515
|
33605.57(22)
|
|
B
|
4873
|
|
4853
|
4840.44(4)
|
|
C
|
4401
|
|
4396
|
4377.60(3)
|
|
|
|
|
|
|
|
|µa|
|
4.10
|
|
2.36
|
|
|
|µb| |
2.90
|
|
1.34
|
|
|
|µc| |
0 (symmetry)
|
|
1.34
|
|
|
* B3PW91/6-311+G(df,pd) dipole moments calculated on molecular structures given by MP2/aug-cc-pVTZ optimization with approximate re CC and CN bond lengths |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table
5. Hydroxyacetonitrile. B3LYP/cc-pVTZ and MP2/cc-pVTZ
Calculated Quartic Centrifugal Distortion Constants (kHz). |
|
|
|
|
|
|
|
|
|
|
|
trans
|
|
|
|
gauche
|
|
|
|
|
B3LYP
|
|
MP2
|
|
B3LYP
|
|
MP2
|
|
|
|
|
|
|
|
|
|
Delta_J
|
|
2.57
|
|
2.64
|
|
3.06
|
|
3.08
|
| Delta_JK |
-
|
58.5
|
-
|
59.7
|
-
|
63.9
|
-
|
65.0
|
| Delta_K |
|
1048.
|
|
1035.
|
|
950.
|
|
956.
|
| delta_J |
|
0.529
|
|
0.554
|
|
0.652
|
|
0.664
|
| delta_K |
|
15.6
|
|
16.1
|
|
17.7
|
|
18.0
|
|
|
|
|
|
|
|
|
|
D_J
|
|
2.51
|
|
2.58
|
|
3.00
|
|
3.01
|
| D_JK | -
|
58.2
|
-
|
59.4
|
-
|
63.5
|
-
|
64.6
|
| D_K |
|
1048.
|
|
1035.
|
|
950.
|
|
956.
|
d_1
|
-
|
0.528
|
-
|
0.554
|
-
|
0.652
|
-
|
0.664
|
d_2
|
-
|
0.0284
|
-
|
0.0303
|
-
|
0.0340
|
-
|
0.0348
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] G.Cazzoli, D.G.Lister, and A.M.Mirri, J.Chem.Soc. Faraday Trans. II 69,569(1973).
|
|
|
|
|
|
|
|
|
|
|
|
|
L.Margulès, R.A.Motiyenko, and J.C.Guillemin, Abstract TI13, 68th OSU International Symposium on Molecular Spectroscopy, 2013.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3CN |
FCH2CN |
ClCH2CN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HOCH2CN.html |
|
|
|
|
|
|
Posted 12 April 2013 |
|
|
|
|
|
|
Last Modified 22 Dec 2013
|
|
|
|
|
|
|
|
|
|
|


