|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(NH2)2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Hydrazine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the nitrogen
nqcc's in hydrazine was made on an equilibrium structure derived ab
initio by Demaison et al. [1]. These are compared in Tables 1 and
2 respectively with the experimental
nqcc's of Harmony and Baron [2] in (ND2)2 and Kasuya [3] in (NH2)2. Structure parameters are
given in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, Subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. RMS is the root mean square difference
between calculated and experimental diagonal nqcc's (percentage of the
average of the magnitudes of the experimental nqcc's). RSD is the
calibration residual standard deviation of the B3PW91/6-311+G(df,pd) model
for calculation of the nitrogen nqcc's, which may be taken as an estimate of the uncertainty in the calculated nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc's in (ND2)2 (MHz). Calculation was made on the re structure [1]. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [2] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
|
4.198 |
|
4.23(4) |
|
|
|
Xbb |
- |
1.963 |
- |
1.98(5) |
|
|
|
Xcc |
- |
2.235 |
- |
2.25(5) |
|
|
|
|Xab| * |
|
1.152 |
|
|
|
|
|
|Xac| |
|
0.653 |
|
|
|
|
|
|Xbc| |
|
3.378 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.028 (0.98 %) |
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.236 |
|
|
|
|
|
Xyy |
|
4.407 |
|
|
|
|
|
Xzz |
- |
5.643 |
|
|
|
|
|
ETA |
|
0.562 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* The algebraic sign of the product XabXacXbc is negative. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen
nqcc's in (NH2)2 (MHz). Calculation was made on the re structure [1]. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [3] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
|
4.100 |
|
4.09 |
|
|
|
Xbb |
- |
1.963 |
|
|
|
|
|
Xcc |
- |
2.136 |
|
|
|
|
|
|Xab| * |
|
1.348 |
|
|
|
|
|
|Xac| |
|
1.025 |
|
|
|
|
|
|Xbc| |
- |
3.304 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* The algebraic sign of the product XabXacXbc is negative. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3. Molecular structure parameters, re [1] (Å
and degrees). |
|
|
re = CCSD(T)/V5Z + CCSD(T)/wCVQZ(ae) - CCSD(T)/wCVQZ(fc) |
|
|
|
|
|
|
|
|
|
|
|
|
Point Group: C2 |
Z-Matrix |
|
|
|
|
|
|
|
|
|
|
|
|
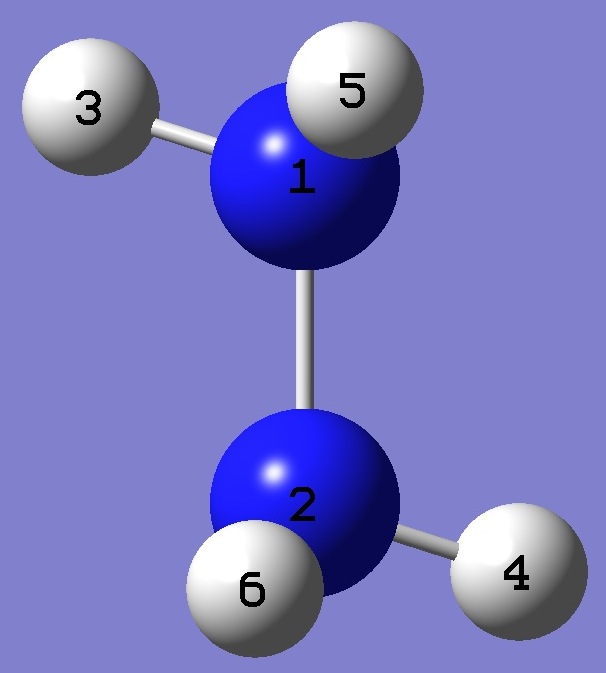 |
N
N 1 B1
H 1 B2 2 A1
H 2 B2
1 A1
3 D1
H 1 B3
2 A2
4 D2
H 2 B3
1 A2
3 D2
B1 1.4324
B2 1.0089
B3 1.0119
A1 107.180
A2 111.576
D1 151.701
D2 -90.759 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] J.Demaison, M.Herman, J.Liévin, L.Margulès, and H.Møllendal, J.Mol.Spectrosc. 244,160(2007). |
|
|
[2] M.D.Harmony and P.A.Baron, J.Mol.Struct. 38,1(1977). |
|
|
[3] T.Kasuya, Sci. Papers Inst. Phys. Chem. Res. 56,1(1962). |
|
|
|
|
|
|
|
|
|
|
|
|
S.Tsunekawa, J.Phys.Soc.Japan 41,2077(1976). ro structure. |
|
|
"Molecular Structure of Hydrazine as
Studied by Gas Electron Diffraction" K.Kohata, T.Fukuyama, and
K.Kuchitsu, J.Phys.Chem. 86,602(1982). |
|
|
"Rotational Isomerism as Studied by Nuclear Quadrupole Coupling. Theoretical and experimental 14N
Chi tensors for hydrazine, methylhydrazine, and 1,2-dimethylhydrazine."
K.Yamanouchi, M.Sugie, H.Takeo, C.Matsumura, and K.Kuchitsu,
J.Mol.Struct. 126,321(1985). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2CHO |
NH2SH |
NF3 |
O=C(NH2)2 |
|
|
NH3 |
NF2H |
NFH2 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH2NH2.html |
|
|
|
|
|
|
Last
Modified 15 Nov 2007 |
|
|
|
|
|
|
|
|
|
|

