|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
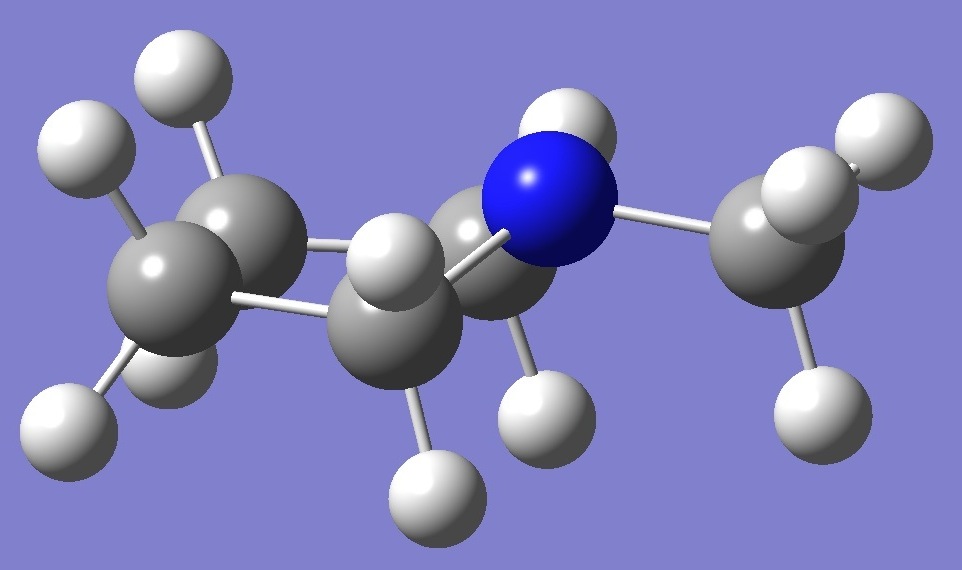
CH3-NC4H8 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
equatorial N-Methylpyrrolidine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation was made here of
the N nqcc tensor in N-Methylpyrrolidine on molecular structures given
by B3P86/6-31G(d,p) and B3P86/6-31G(3d,3p) optimization.
These nqcc's are compared with experimental values [1] in Table 1. Structure parameters are
given in Table 2, rotational constants and dipole moments in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1,
subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor.
Ø (degrees) is the angle between its subscripted
parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean
square difference between calculated and experimental diagonal nqcc's
(percent of average absolute experimental nqcc). RSD is the
calibration residual standard deviation of the B3PW91/6-311+G(df,pd)
model for calculation of nitrogen nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc's in N-Methylpyrrolidine
(MHz). Calculation was made on (1) B3P86/6-31G(d,p) and (2)
B3P86/6-31G(3d,3p) optimized molecular structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2) |
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
2.584 |
|
2.593 |
|
2.729(119)
|
|
|
Xbb |
|
2.670 |
|
2.688 |
|
2.494(80)
|
|
|
Xcc |
- |
5.255 |
- |
5.281 |
-
|
5.223
|
|
|
|Xac| |
|
0.593 |
|
0.565 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.133 (3.8 %)
|
|
0.141 (4.0 %)
|
|
|
|
|
RSD
|
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.629 |
|
2.633 |
|
|
|
|
Xyy |
|
2.670 |
|
2.688 |
|
|
|
|
Xzz |
- |
5.299 |
- |
5.322 |
|
|
|
|
ETA |
|
0.0078 |
|
0.0103 |
|
|
|
|
Øz,a |
|
94.30 |
|
94.08 |
|
|
|
|
Øa,N-Me |
|
13.58 |
|
13.74 |
|
|
|
|
Øz,N-Me |
|
107.88 |
|
107.83 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
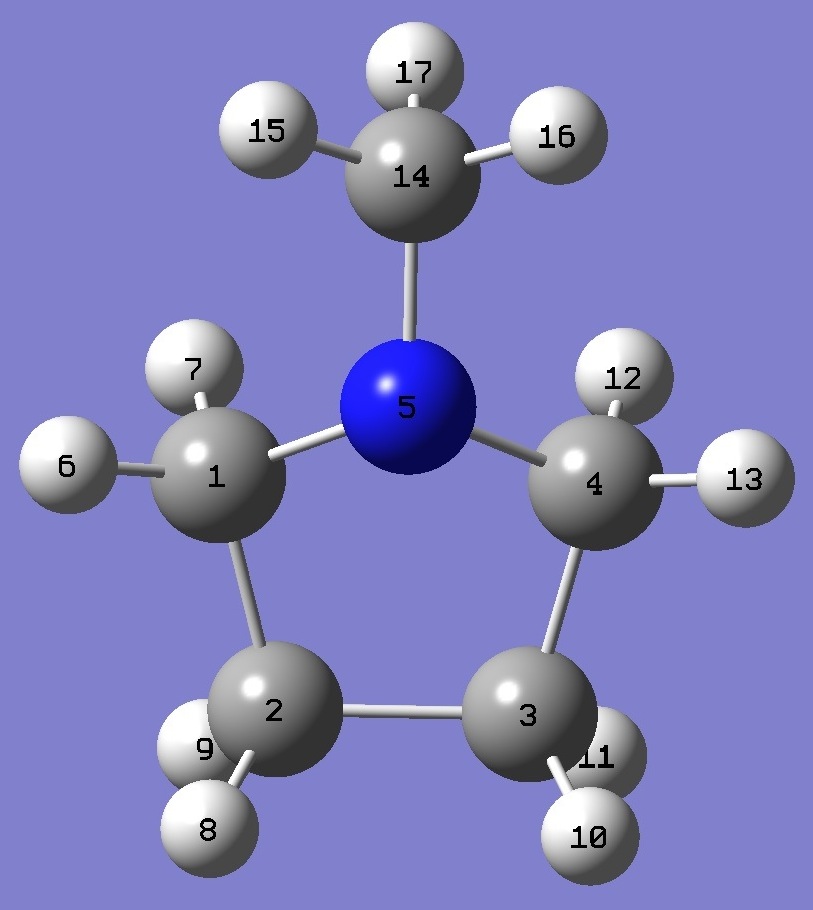
| Table 2.
N-Methylpyrrolidine. Selected structure parameters (Å
and degrees). ropt(1) = B3P86/6-31G(d,p) and ropt(2)
= B3P86/6-31G(3d,3p) optimized molecular structures.
Complete structures are given here
in Z-matrix format. |
| |
|
|
|
|
|
ropt(1) |
ropt(2) |
|
|
|
| NC(14) |
1.4418 |
1.4414 |
| NC(1,4) |
1.4542 |
1.4543 |
| C(14)NC(1,4) |
114.04 |
114.04 |
| C(1)NC(4) |
104.40 |
104.59 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3. N-Methylpyrrolidine.
Rotational constants (MHz)and dipole moments (D). ropt(1)
= B3P86/6-31G(d,p) and ropt(2) = B3P86/6-31G(3d,3p)
optimized molecular structures.
|
| |
|
|
|
|
|
|
ropt(1) |
ropt(2) |
Expt [1]
|
|
|
|
|
|
|
A |
6672.8 |
6674.5 |
6636.726(1)
|
|
B |
3168.0 |
3172.3 |
3154.4941(5)
|
|
C |
2358.3 |
2359.0 |
2351.6616(4)
|
|
|
|
|
|
|
|µa| |
0.12 |
0.13 |
0.08(1)
|
|
|µb| |
0 (sym) |
0 (sym) |
0.0
|
|
|µc| |
0.56 |
0.56 |
0.566(3)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] W.Caminati and F.Scappini, J.Mol.Spectrosc. 117,184(1986).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyrrolidine |
Nicotine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NMePRL.html |
|
|
|
|
|
|
Last
Modified 29 June 2010 |
|
|
|
|
|
|
|
|
|
|

