|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(CH3CH2)3N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
Triethylamine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen nqcc's in the minimum
energy C3 conformer of triethylamine were
determined by Nguyen et al. [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 14N
nqcc tensors was made here on molecular structures derived by
MP2/6-311+G(d,p) and MP2/6-311+G(df,pd) optimizations (Note:
Optimizations were undertaken using the NoSymm keyword. The
results were very near C3 symmetry and were,
therefore, symmetrized to C3.
B3PW91/6-311+G(df,pd) electric field gradients were calculated on the
symmetrized structures.). Calculated and experimental nqcc's are
compared in
Table 1. Structure parameters are given in Table 2, rotational
constants in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
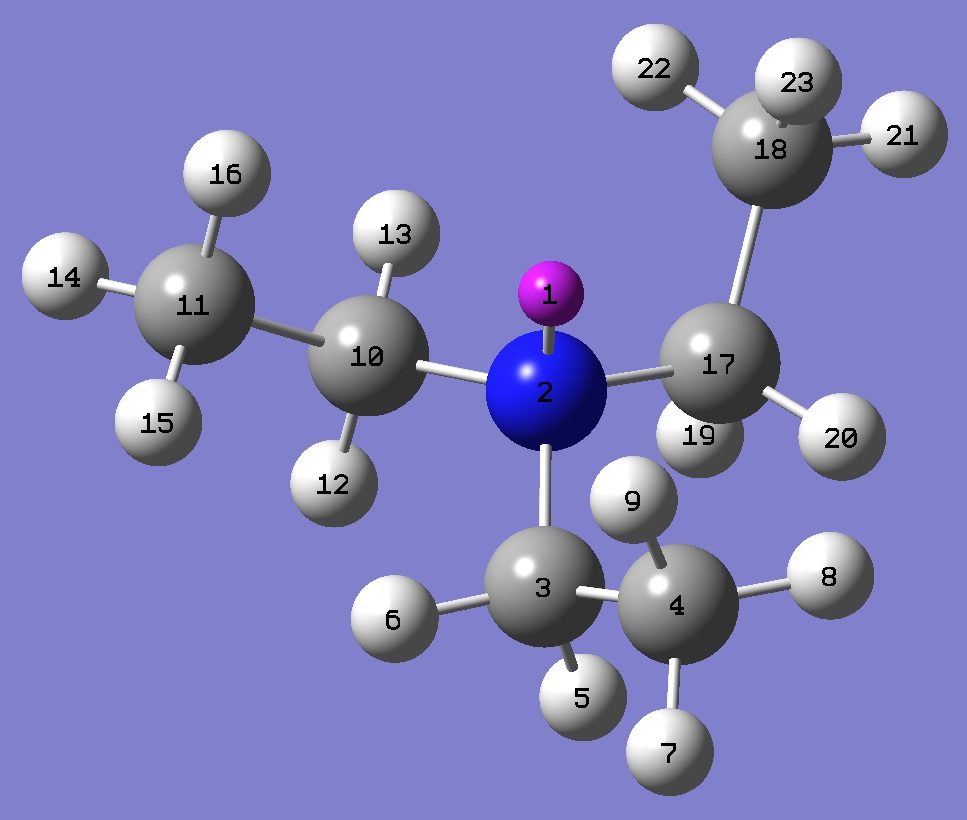
Table 1. 14N nqcc's in
Triethylamine
(MHz). Calculation was made on the symmetrized (1)
MP2/6-311+G(d,p)
and (2) MP2/6-311+G(df,pd) optimized molecular structures. Atomic
numbering is shown below. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Species
|
|
Parameter
|
|
Calc. (1) |
|
Calc. (2) |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Normal |
|
Xcc |
- |
5.253 |
- |
5.244 |
- |
5.2444(7) |
|
|
|
|
|
|
|
|
|
|
|
|
|
13C(3) |
|
Xcc |
- |
5.253 |
- |
5.243 |
- |
5.2660(40) |
|
|
|
|
Xbb - Xaa |
|
0.000 |
|
0.000 |
|
|
|
|
|
|
|Xac| |
|
0.029 |
|
0.028 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
13C(4) |
|
Xcc |
- |
5.253 |
- |
5.243 |
- |
5.947(98) |
|
|
|
|
Xbb - Xaa |
|
0.000 |
|
0.000 |
- |
0.257(19) |
|
|
|
|
|Xac| |
|
0.048 |
|
0.048 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 2.
Triethylamine. Selected structure
parameters (Å and
degrees). Complete structures are given here
in Z-matrix format. |
| |
|
|
|
|
 |
ropt (1) =
MP2/6-311+G(d,p) optimization, C3 |
| ropt (2) =
MP2/6-311+G(df,pd) optimization, C3 |
| |
|
|
|
ropt (1) |
ropt (2) |
|
|
|
| NC(3) |
1.4660 |
1.4598 |
| C(3)NC(10) |
110.59 |
110.71 |
| C(3)C(4) |
1.5256 |
1.5209 |
| NC(3)C(4) |
112.48 |
112.57 |
| XNC(3)C(4) |
- 39.17 |
- 39.20 |
|
|
|
| X is dummy atom |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
| Table 3.
Triethylamine.
Rotational constants (GHz). |
| |
|
|
|
|
|
|
ropt (1) =
MP2/6-311+G(d,p) optimization, C3 |
|
ropt (2) =
MP2/6-311+G(df,pd) optimization, C3 |
| |
|
|
|
|
|
|
Species |
|
Calc. ropt (1) |
Calc. ropt (2) |
Expt. [1] |
|
|
|
|
|
|
|
Normal |
A |
2.3199 |
2.3351 |
2.314873978(11) |
|
|
B |
2.3199 |
2.3351 |
2.314873978(11) |
|
|
C |
1.3260 |
1.3349 |
--------- |
|
|
|
|
|
|
|
13C(2) |
A |
2.3179 |
2.3331 |
2.31293315(34) |
|
|
B |
2.2976 |
2.3127 |
2.29283301(33) |
|
|
C |
1.3193 |
1.3282 |
1.32681(96) |
|
|
|
|
|
|
|
13C(3) |
A |
2.3181 |
2.3333 |
2.31302815(28) |
|
|
B |
2.2560 |
2.2708 |
2.25126785(40) |
|
|
C |
1.3054 |
1.3142 |
1.32989(18) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] H.V.L.Nguyen, R.Kannengiesser,
and W.Stahl, PCCP 14,11753(2012) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NH3 |
CH3NH2 |
CH3CH2NH2 |
Diethylamine
|
|
Dimethylamine |
Trimethylamine |
Quinuclidine |
NF3 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Triethylamine.html |
|
|
|
|
|
|
Last
Modified 16 July 2012 |
|
|
|
|
|
|
|
|
|
|

