| |
|||||||
| Table 1. Nitrogen nqcc's in antiperiplanar cyclopropyl-methylisocyanide (MHz). Calculation was made
on CCSD/cc-pVTZ ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14N | Xaa | 0.067 |
|||||
| Xbb | 0.117 |
||||||
| Xcc | - |
0.184 |
|||||
| |Xab| | 0.297 |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | - |
0.206 |
|||||
| Xyy | - |
0.184 |
|||||
| Xzz | 0.390 |
||||||
| ETA |
- |
0.0574 |
|||||
| Øz,a |
132.61 |
||||||
| Øa,NC | 129.15 |
||||||
| Øz,NC | 3.46 |
||||||
| |
|||||||
| |
|||||||
| Table 2. Nitrogen nqcc's in synclinal cyclopropyl-methylisocyanide (MHz). Calculation was made
on CCSD/cc-pVTZ ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14N | Xaa | 0.233 |
|||||
| Xbb | - |
0.051 |
|||||
| Xcc | - |
0.182 |
|||||
| Xab | 0.270 |
||||||
| Xac | 0.049 |
||||||
| Xbc | 0.015 |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | - |
0.219 |
|||||
| Xyy | - |
0.182 |
|||||
| Xzz | 0.401 |
||||||
| ETA |
- |
0.0942 |
|||||
| Øz,NC | 1.87 |
||||||
| |
|||||||
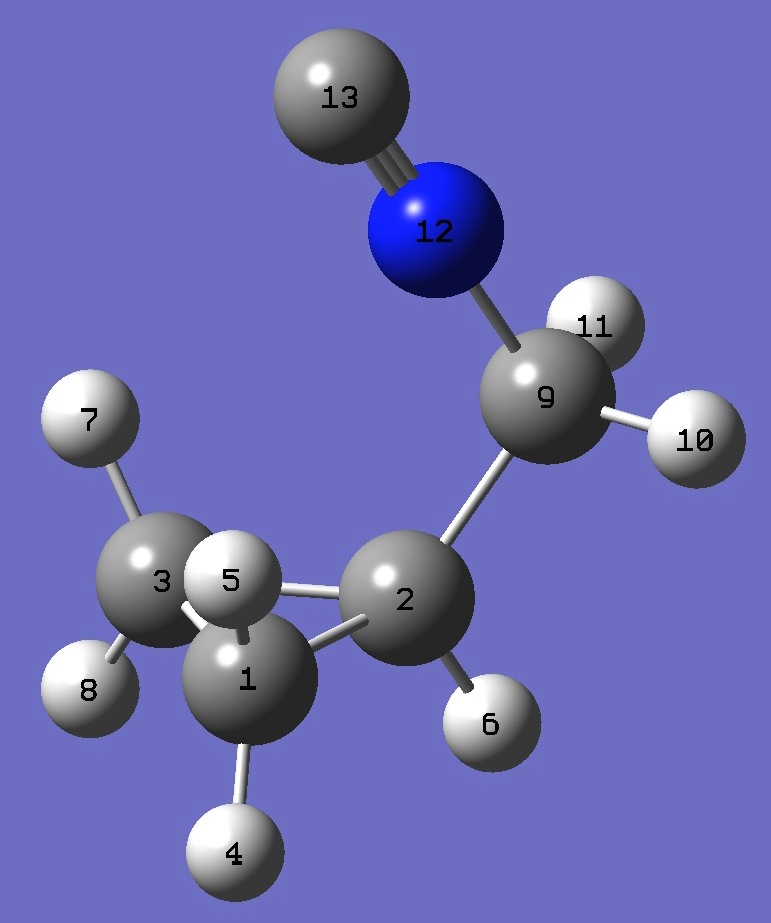
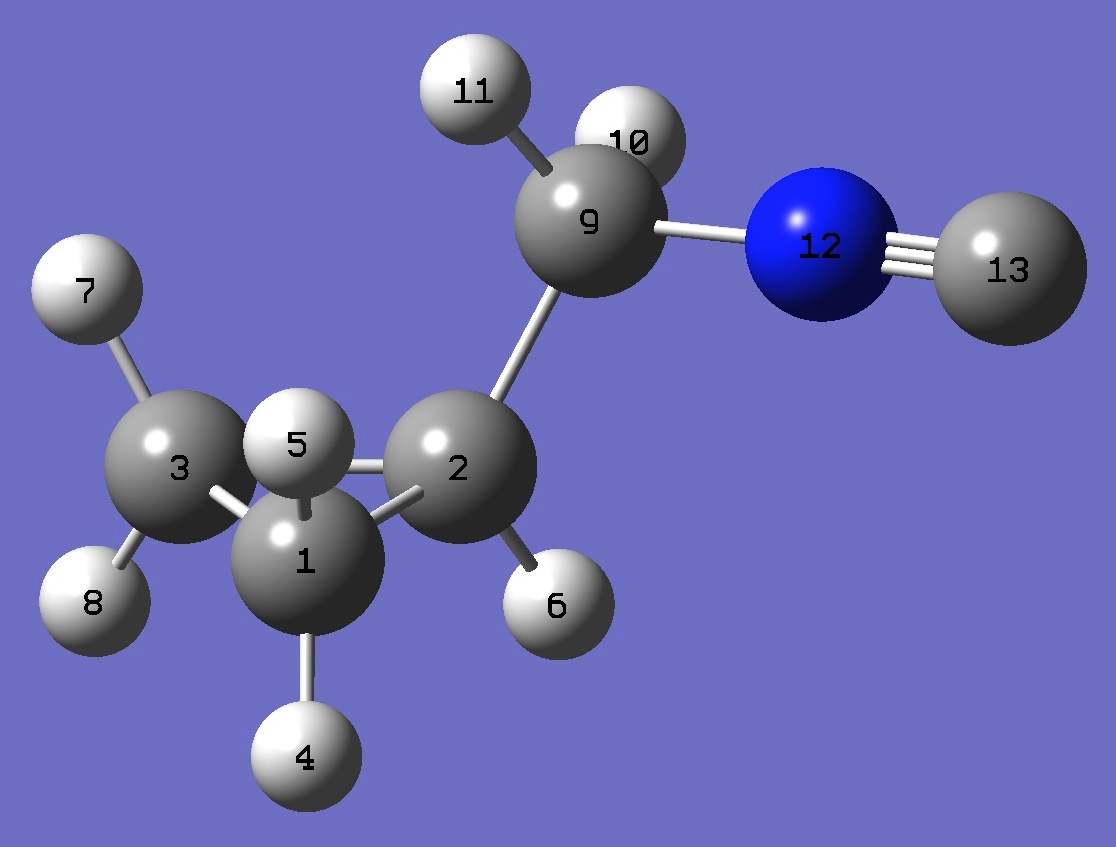
| Table 3. Cyclopropyl-methylisocyanide CCSD/cc-pVTZ optimized structure parameters [1] (Å and degrees). Atomic numbering is shown above. | |||||
| |
|
||||
| |
|||||
| # B3PW91/6-311+G(df,pd) prop scf=tight Cyclopropyl-methylisocyanide 0 1 C C 1 B1 C 2 B2 1 A1 H 1 B3 2 A2 3 D1 0 H 1 B4 2 A3 3 D2 0 H 2 B5 1 A4 3 D3 0 H 3 B6 2 A5 1 D4 0 H 3 B7 2 A6 1 D5 0 C 2 B8 1 A7 3 D6 0 H 9 B9 2 A8 1 D7 0 H 9 B10 2 A9 1 D8 0 N 9 B11 2 A10 1 D9 0 C 12 B12 9 A11 2 D10 0 |
|||||
| antiperiplanar |
synclinal |
||||
| B1 1.50145866 B2 1.50145866 B3 1.07946880 B4 1.08005856 B5 1.08150724 B6 1.08005856 B7 1.07946880 B8 1.51341907 B9 1.08988332 B10 1.08988332 B11 1.42862123 B12 1.16930468 A1 60.34167438 A2 117.76580907 A3 117.66948089 A4 116.46533273 A5 117.66948089 A6 117.76580907 A7 121.38919042 A8 110.40581259 A9 110.40581259 A10 112.33706505 A11 178.81983388 D1 108.34171848 D2 -106.66016651 D3 -106.82220276 D4 106.66016651 D5 -108.34171848 D6 110.77484300 D7 84.18845953 D8 -156.32418583 D9 -36.06786315 D10 0.00000000 |
B1 1.50131653 B2 1.50384407 B3 1.07917063 B4 1.08076689 B5 1.08086205 B6 1.08095315 B7 1.07928658 B8 1.50551753 B9 1.08952077 B10 1.09023076 B11 1.43173206 B12 1.16931237 A1 60.21948209 A2 117.93191854 A3 117.38471772 A4 116.98659776 A5 117.70252117 A6 117.91568964 A7 118.91507695 A8 111.15963726 A9 110.11397519 A10 111.64721913 A11 178.72574733 D1 108.10394109 D2 -107.88196261 D3 -107.40598838 D4 107.55346181 D5 -108.18243445 D6 107.84985817 D7 -157.23883489 D8 -37.62224613 D9 82.27360804 D10 3.57991398 |
||||