|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[C(=O)H]2NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Formimide
|
|
|
(Diformamide, N-Formylformamide)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
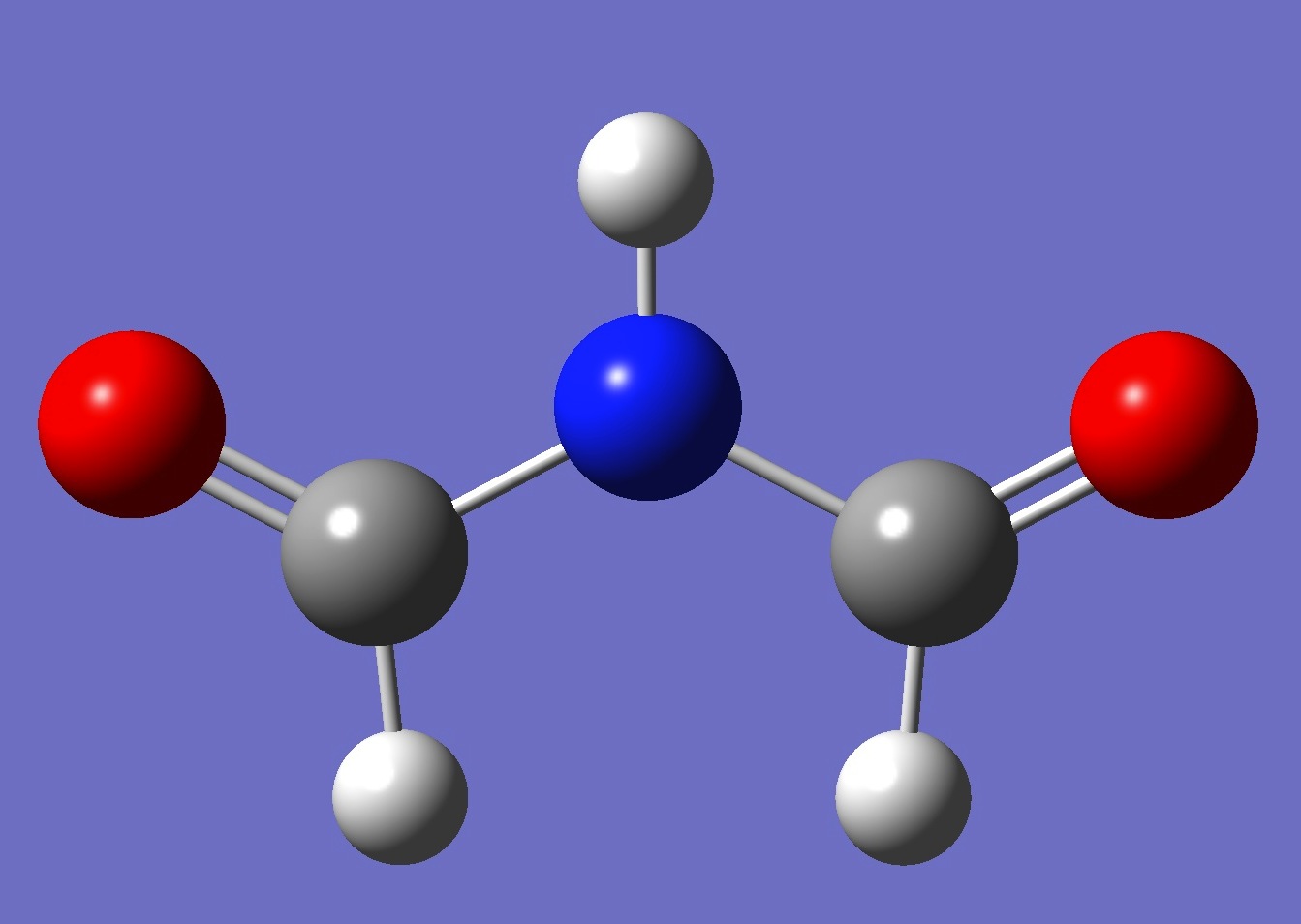
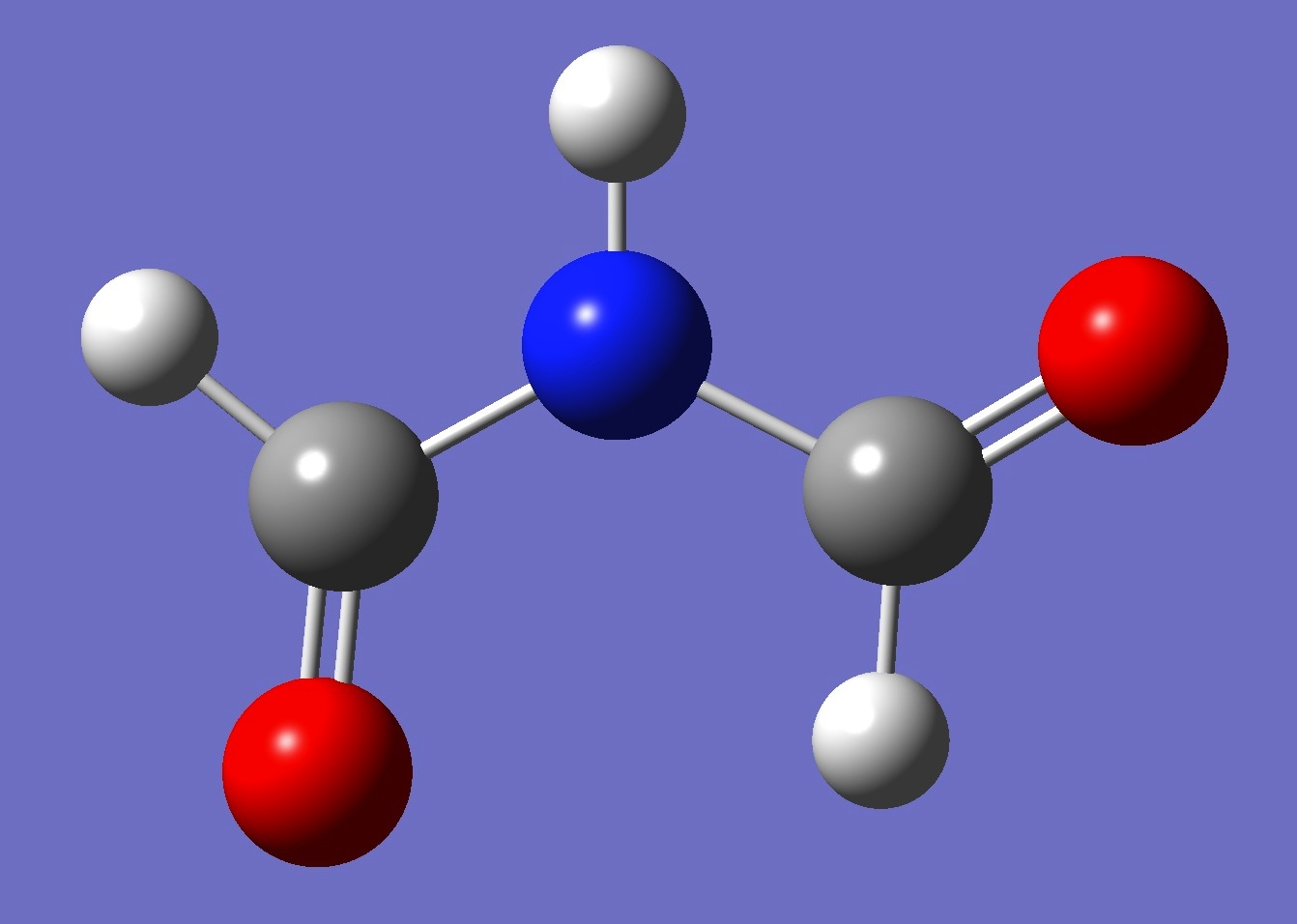
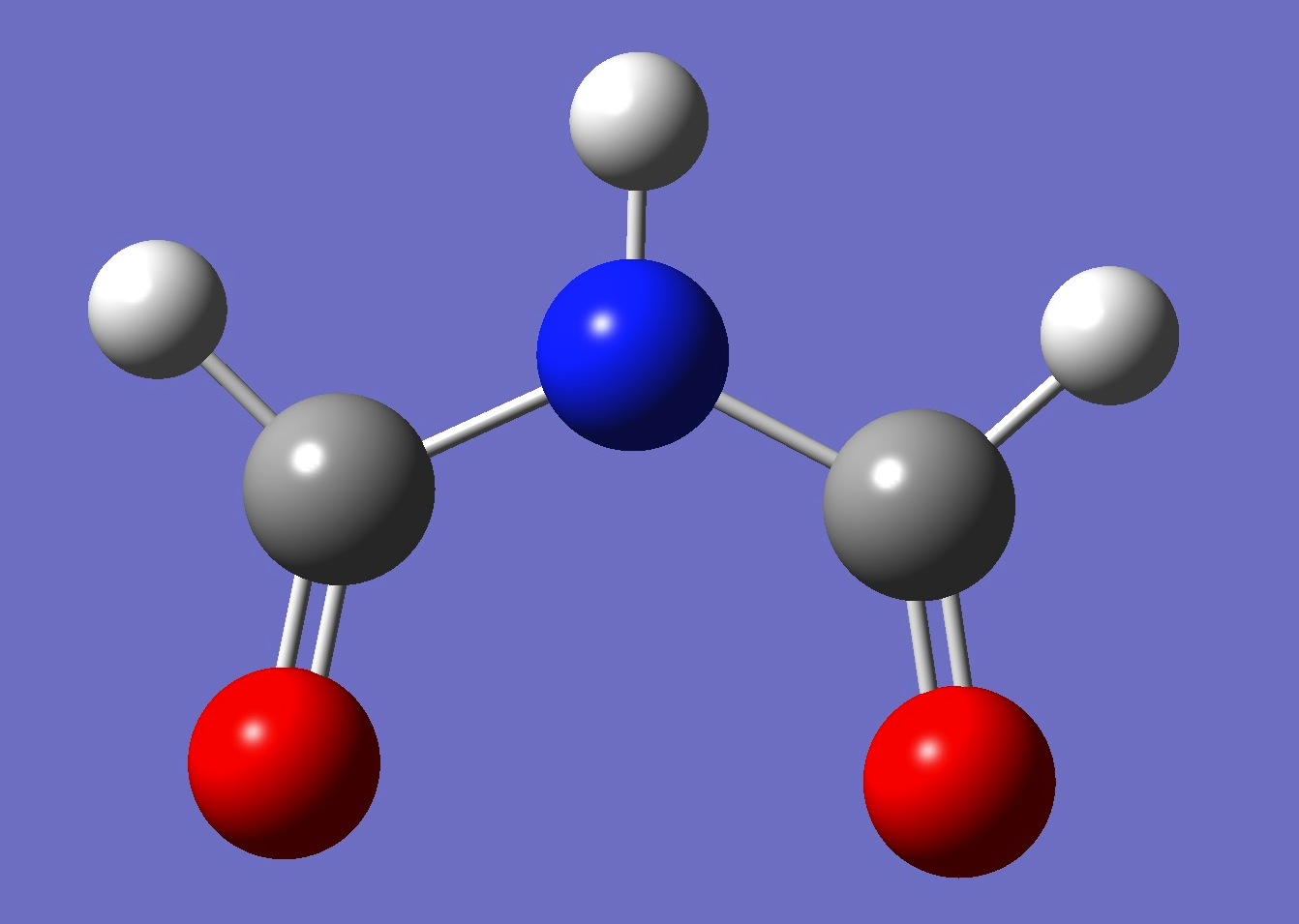
Possible planar conformers of formimide, and MP2/aug-cc-pVTZ energies, are shown below:
|
|
|
|
|
|
|
|
|
|
|
|
|
cis-cis
|
|
cis-trans
|
|
trans-trans
|
|
|
|
|
|
| 
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 0
|
|
3.1 kJ/mol
|
|
26.4 kJ/mol
|
|
|
|
|
|
|
|
|
|
|
|
|
The microwave spectrum of the cis-trans conformer was investigated by Steinmetz [1], Nitrogen nqcc's were determined.
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation was made here of the 14N nqcc tensors on ropt
molecular structures given by MP2/aug-cc-pVTZ and HF/aug-cc-pVTZ
optimizations. These calculated nqcc's are given
in Tables 1 and 2.
Structure
parameters, along with corresponding rotational constants and dipole
moments, are given in Table 3. Centrifugal distortion constants
are given in Table 4. |
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1 and 2, subscripts a,b,c
refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. RMS is root mean square difference between calculated and experimental nqcc's. RSD is the calibration residual
standard deviation of
the B3PW91/6-311+G(df,pd) model for calculation of the nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc's in cis-cis Formimide (MHz).
Calculation was made on MP2 and HF ropt molecular structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /MP2
|
|
Calc /HF |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
1.367
|
|
1.300
|
|
|
|
|
Xbb |
|
1.464
|
|
1.496
|
|
|
|
|
Xcc |
-
|
2.830
|
-
|
2.796
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %)
|
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.367
|
|
1.300
|
|
|
|
|
Xyy |
|
1.464
|
|
1.496
|
|
|
|
|
Xzz |
-
|
2.830
|
-
|
2.796
|
|
|
|
|
ETA |
|
0.034
|
|
0.070
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N
nqcc's in cis-trans Formimide (MHz).
Calculation was made on MP2 and HF ropt molecular structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /MP2
|
|
Calc /HF |
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
1.437
|
|
1.372
|
|
1.88 (11)
|
|
|
Xbb |
|
1.580
|
|
1.594
|
|
1.53(7)
|
|
|
Xcc |
-
|
3.018
|
-
|
2.966
|
-
|
3.41(7)
|
|
|
Xab |
|
0.064
|
|
0.056
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.343 (15. %)
|
|
0.391 (17. %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.413
|
|
1.359
|
|
|
|
|
Xyy |
|
1.605
|
|
1.607
|
|
|
|
|
Xzz |
-
|
3.018
|
-
|
2.966
|
|
|
|
|
ETA |
|
0.063
|
|
0.084
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3. Formimide, ropt structure parameters (Å and degrees), rotational constants (MH), and electric dipole moments (D).
|
|
|
|
|
|
|
_________cis-cis__________
|
________cis-trans_________ |
| MP2 |
HF
|
MP2 |
HF
|
|
|
|
|
|
NH
|
1.0126
|
0.9969
|
1.0102
|
0.9942
|
NC(1)
|
1.3818
|
1.3691
|
1.3899
|
1.3797
|
| C(1)Oc
|
1.2113
|
1.1802
|
1.2124
|
1.1811
|
| C(1)Hc
|
1.0983
|
1.0890
|
1.0932
|
1.0832
|
NC(2)
|
1.3818
|
1.3691 |
1.3847
|
1.3700
|
| C(2)Ot
|
1.2113
|
1.1802 |
1.2119
|
1.1817
|
C(2)Ht
|
1.0983
|
1.0890 |
1.0984
|
1.0889
|
HNC(1)
|
118.13
|
117.83
|
116.63
|
116.42 |
| NC(1)Oc |
123.75
|
123.72
|
123.37
|
122.25
|
| NC(1)Hc |
112.69
|
113.41
|
112.80
|
113.49
|
| HNC(2) |
118.13
|
117.83 |
119.23
|
119.10
|
| NC(2)Ot |
123.75
|
123.72 |
124.59
|
124.82
|
| NC(2)Ht |
112.69
|
113.41 |
112.12
|
112.40
|
|
|
|
|
|
A
|
43229.
|
44472.
|
21650.
|
22512.
|
B
|
2460.
|
2534.
|
3009.
|
3087.
|
C
|
2327.
|
2397.
|
2642.
|
2715.
|
Expt A,B, and C are respectively 21490.671(18), 3029.943(2), and 2655.748(2) MHz [1]
|
|
|
|
|
|
|µa|
|
0
|
0
|
1.12
|
1.02
|
| |µb| |
1.58
|
1.52
|
2.73
|
2.58
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 4. Formimide. Centrifugal Distortion Constants (kHz). Calc = B3LYP/cc-pVTZ.
|
|
|
|
|
|
|
_________cis-cis__________
|
________cis-trans_________ |
| Calc |
Expt
|
Calc |
Expt
|
|
|
|
|
|
Delta_J
|
0.199
|
|
0.743 |
|
| Delta_JK |
- 3.56
|
|
- 11.6 |
|
| Delta_K |
357.
|
|
149.
|
|
| delta_J |
0.0143
|
|
0.144
|
|
| delta_K |
1.08
|
|
3.17
|
|
|
|
|
|
|
D_J
|
0.198
|
|
0.720
|
|
D_IK
|
- 3.55
|
|
- 11.5
|
|
D_K
|
357.
|
|
149.
|
|
d_1
|
- 0.0143
|
|
- 0.144
|
|
d_2
|
- 0.00044
|
|
- 0.00738
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] W.E.Steinmetz, JACS 95(9),2777(1973),
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Formamide
|
Acetamide
|
see Amides
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
formimide.html |
|
|
|
|
|
|
Last
Modified 20 April 2015 |
|
|
|
|
|
|
|
|
|
|


