| |
|||||||
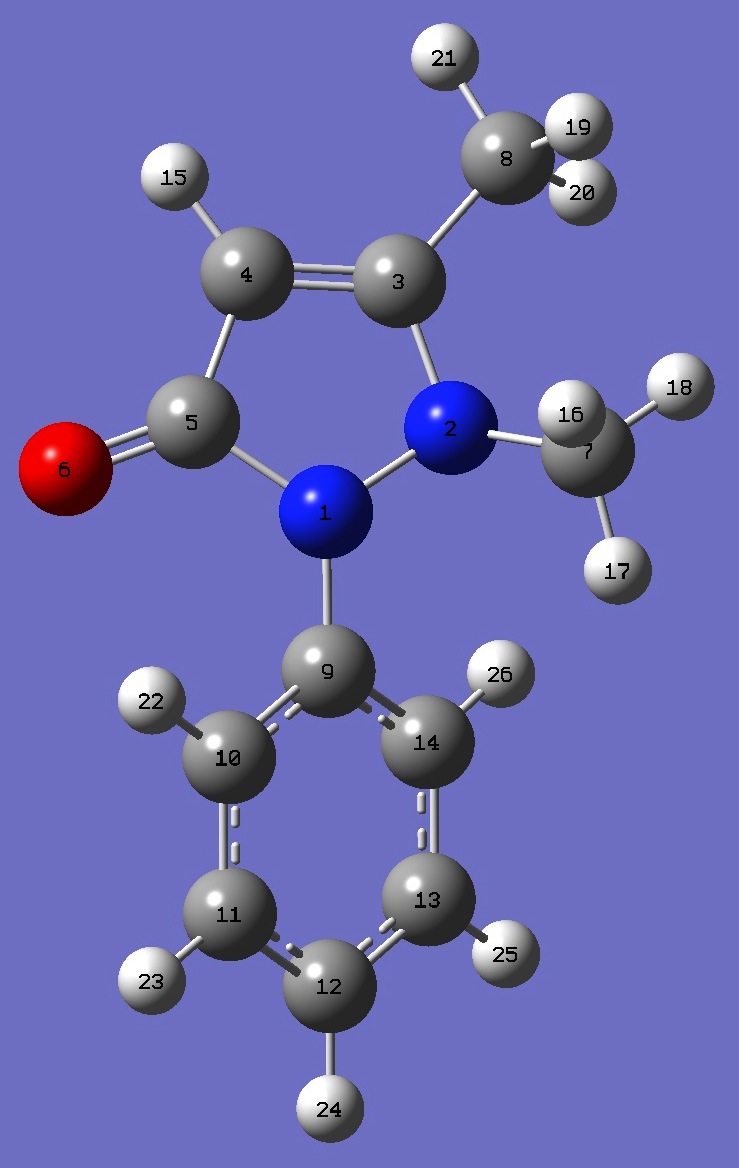
| Table 1. Nitrogen nqcc's in Phenazone (MHz). Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt [1] |
||||||
| |
|||||||
| 14N(1) * |
Xaa | 2.404 |
2.480(10) |
||||
| Xbb | 2.645 |
2.6498(98) |
|||||
| Xcc | - |
5.049 |
- |
5.1298(98) |
|||
| Xab | - |
1.508 |
|||||
| Xac | - |
0.301 |
|||||
| Xbc | 0.090 |
||||||
| RMS |
0.064 (1.9 %) |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | 1.016 |
||||||
| Xyy | 4.046 |
||||||
| Xzz | 5.062 |
||||||
| ETA |
0.598 |
||||||
| Ř ** | 15.16 |
||||||
| |
|||||||
| |
|||||||
| Table 2. Nitrogen nqcc's in Phenazone (MHz). Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt [1] |
||||||
| |
|||||||
| 14N(2) * |
Xaa | 1.849 |
1.780(11) |
||||
| Xbb | 1.146 |
1.100(10) |
|||||
| Xcc | - |
2.995 |
- |
2.880(10) |
|||
| Xab | - |
2.472 |
|||||
| Xac | - |
1.820 |
|||||
| Xbc | - |
2.576 |
|||||
| RMS |
0.082 (4.3 %) |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | 1.283 |
||||||
| Xyy | 4.013 |
||||||
| Xzz | - |
5.296 |
|||||
| ETA |
0.515 |
||||||
| Ř ** | 28.78 |
||||||
| |
|||||||
| Table
3. Phenazone. Rotational Constants (MHz) and Electric
Dipole Moments (D). |
||||
| Calc. |
Expt [1] |
|||
| A |
1432.0 |
1424.59526(71) |
||
| B |
500.3 |
496.56384(24) |
||
| C |
400.6 |
400.108793(96) |
||
| |µa| |
2.26 |
|||
| |µb| | 5.00 |
|||
| |µc| | 0.86 |
|||