| |
|||||||
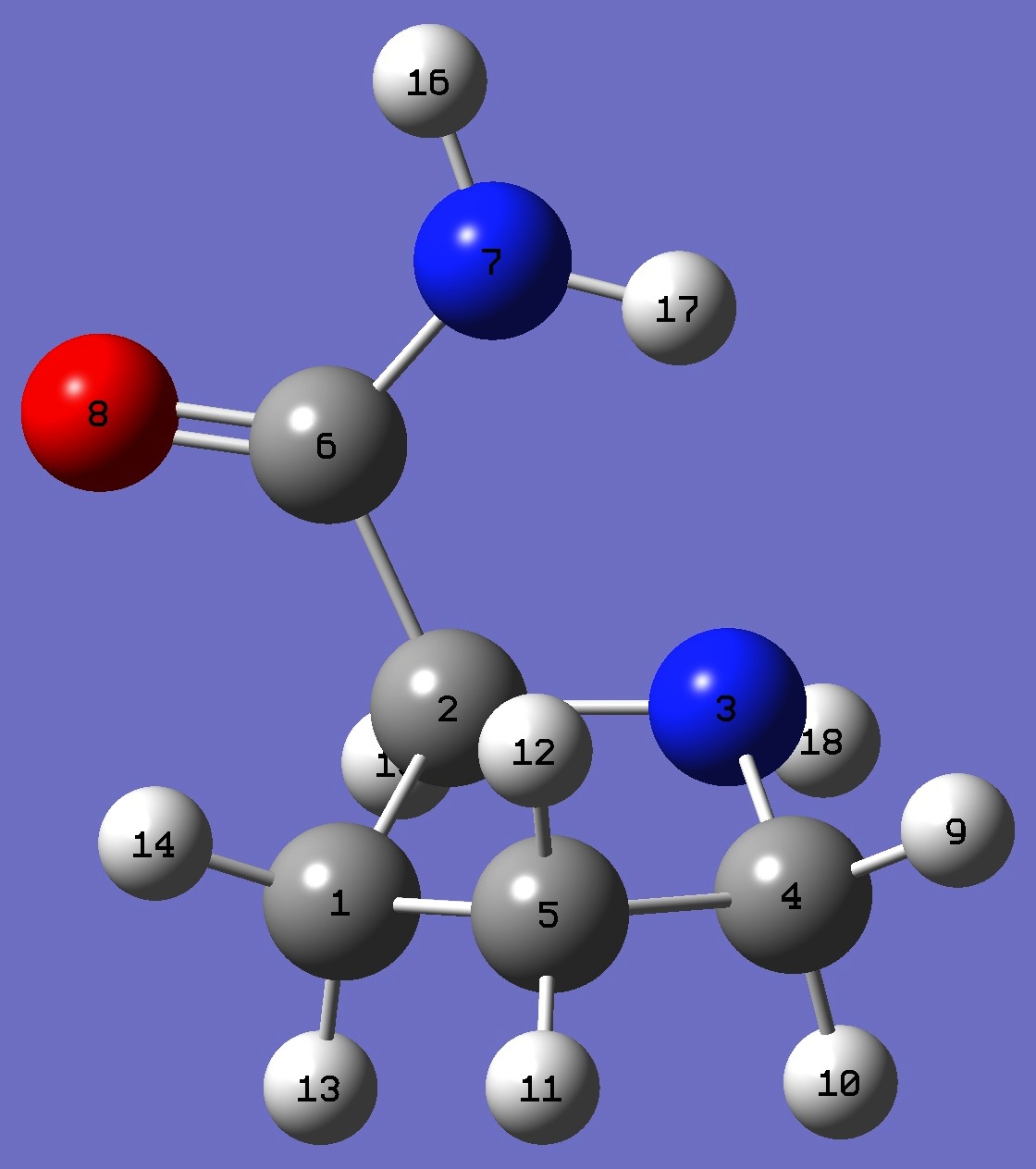
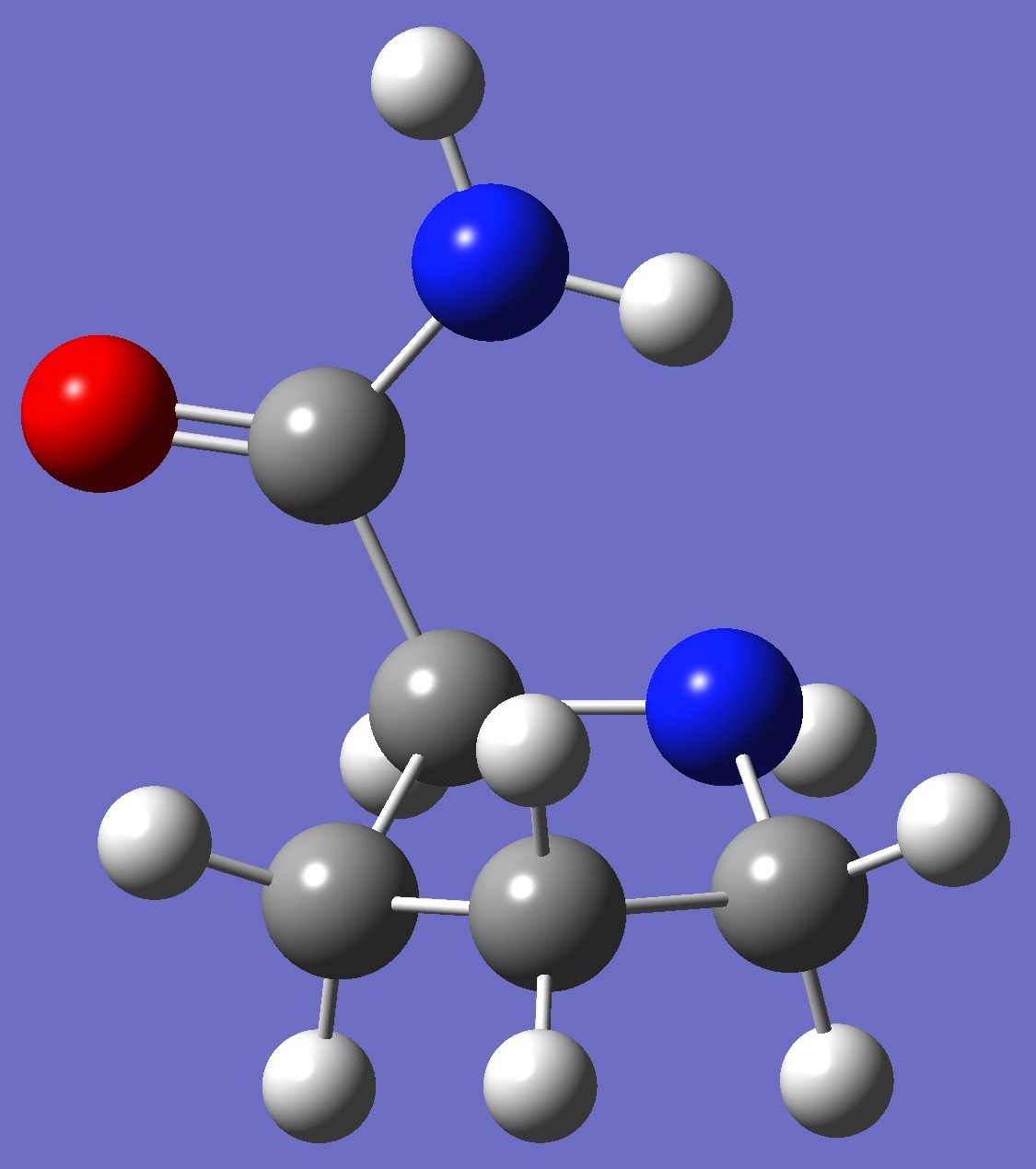
| Table 1. 14Npro nqcc's in Prolinamide (MHz). Calculation was made on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14Npro | Xaa | 1.354 |
|||||
| Xbb | - |
0.722 |
|||||
| Xcc | - |
0.632 |
|||||
| Xab | - |
2.401 |
|||||
| Xac | - |
2.285 |
|||||
| Xbc | - |
2.831 | |||||
| RSD | 0.030 (1.3 %) | ||||||
| |
|||||||
| Xxx | 2.152 |
||||||
| Xyy | 3.036 |
||||||
| Xzz | - |
5.188 |
|||||
| ETA |
0.170 |
||||||
| |
|||||||
| |
|||||||
| Table 2. 14Nam nqcc's in Prolinamide (MHz). Calculation was made on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14Nam | Xaa | 1.030 |
|||||
| Xbb | 0.906 |
||||||
| Xcc | - |
1.936 |
|||||
| Xab | - |
0.411 |
|||||
| Xac | 1.780 |
||||||
| Xbc | 1.799 | ||||||
| RSD | 0.030 (1.3 %) | ||||||
| |
|||||||
| Xxx | 1.376 |
||||||
| Xyy | 2.135 |
||||||
| Xzz | - |
3.511 |
|||||
| ETA |
0.216 |
||||||
| |
|||||||
| Table 4. Prolinamide. Rotational Constants (MHz) and Dipole Moments (D). Rotational constants = B3P86/6-31G(3d,3p). Dipole moments = B3PW91/6-311+G(df,pd) calculation on B3P86/6-31G(3d,3p) opt structure. |
|||
| |
|||
| Calc | Expt [1] |
||
| |
|||
| A |
3758. |
3640.355(89) |
|
| B |
1618. |
1655.717(20) |
|
| C |
1364. |
1386.497(15) |
|
| |µa| |
3.32 |
3.13(4) |
|
| |µb| |
2.38 |
2.21(2) |
|
| |µc| |
0.21 |
0.1(4) |
|
| Table 5. Prolinamide. Nitrogen atomic coordinates, Kraitchman [1] and ropt = B3P86/6-31G(3d,3p) (Å). Kraitchman coordinates are absolute values. |
|||||||
| amino (pro) nitrogen |
amido nitrogen |
||||||
| ropt | Kraitchman |
ropt | Kraitchman | ||||
| a |
0.851 |
0.874(2) |
- |
1.528 |
1.533(1) |
||
| b |
- |
0.986 |
0.999(2) |
- |
1.191 |
1.221(1) |
|
| c |
0.598 |
0.628(2) |
- |
0.596 |
0.539(3) |
||
| NOTE: Kratchman N-N distance is 2.684(2) Å, ropt N-N distance is 2.671 Å. | |||||||