|
| |
|
|
|
|
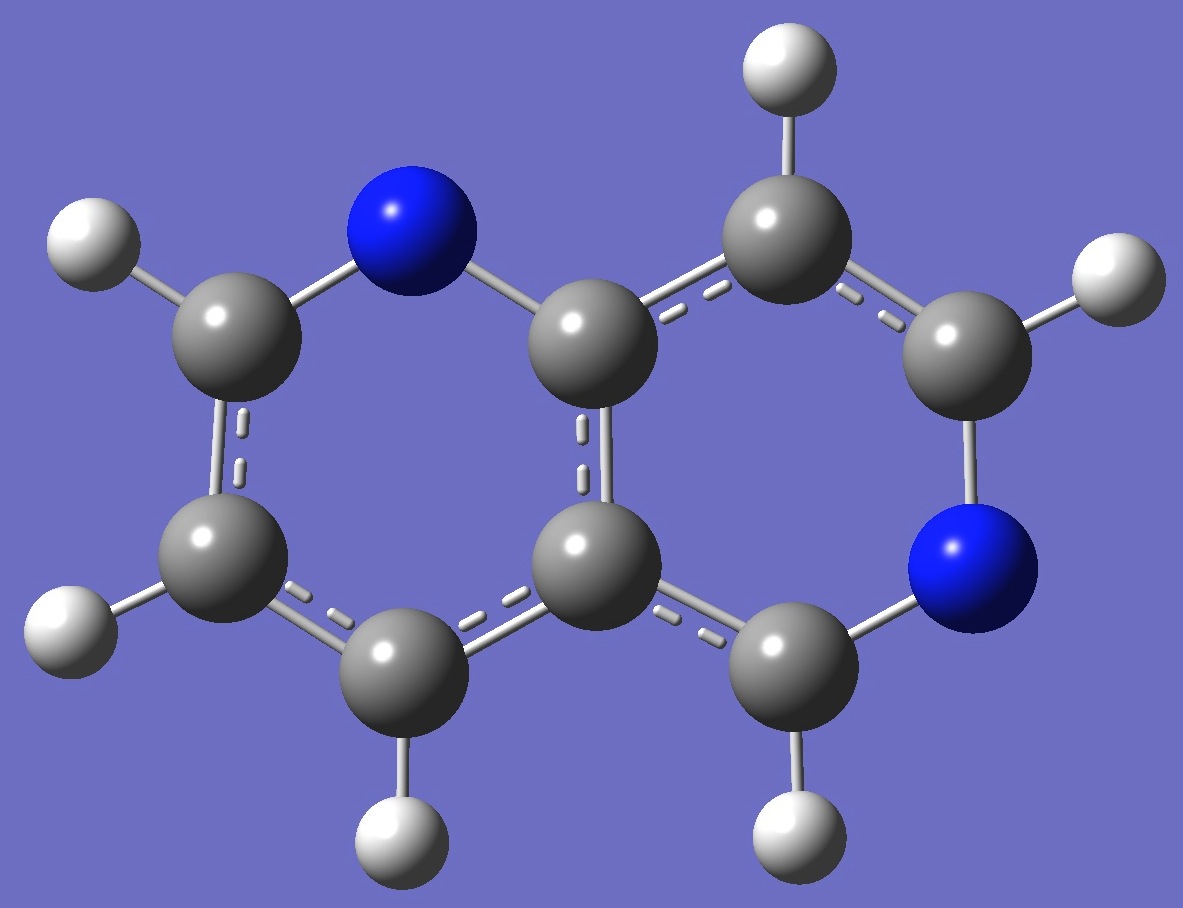
Table 3. 1,6-Naphthyridine. Structure Parameters (Å and degrees).
|
| |
|
|
|
|
|
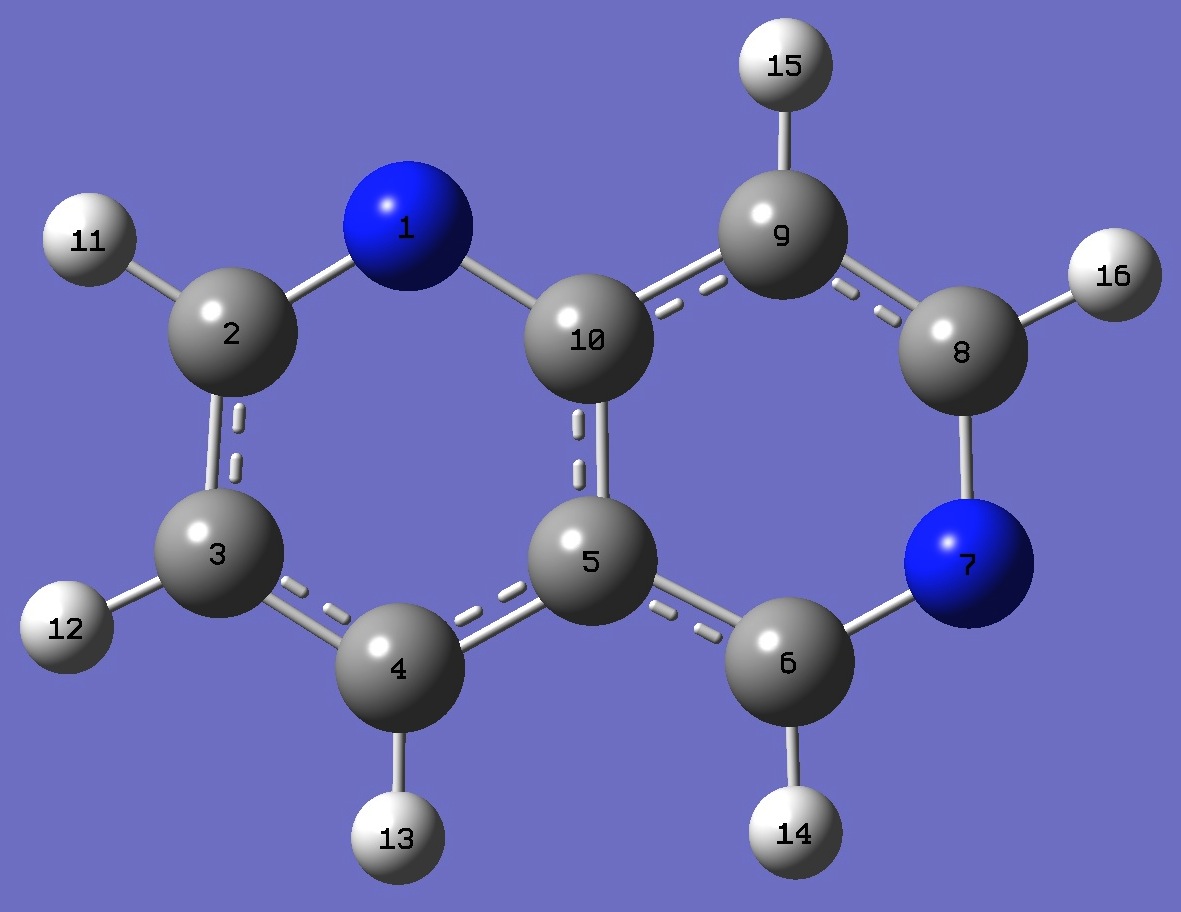
N
C,1,R1
C,2,R2,1,A1
C,3,R3,2,A2,1,D1,0
C,4,R4,3,A3,2,D2,0
C,5,R5,4,A4,3,D3,0
N,6,R6,5,A5,4,D4,0
C,7,R7,6,A6,5,D5,0
C,8,R8,7,A7,6,D6,0
C,5,R9,4,A8,3,D7,0
H,2,R10,1,A9,10,D8,0
H,3,R11,2,A10,1,D9,0
H,4,R12,3,A11,2,D10,0
H,6,R13,5,A12,4,D11,0
H,9,R14,8,A13,7,D12,0
H,8,R15,7,A14,6,D13,0
|
|
|
|
|
|
| |
B3P86/6-31G(d,p) opt
|
B3P86/6-31G(3d,3p) opt
|
|
|
|
|
|
|
R1=1.31492812
R2=1.41633434
R3=1.37137072
R4=1.41250047
R5=1.41753563
R6=1.31289916
R7=1.35874384
R8=1.37342199
R9=1.42205395
R10=1.08932497
R11=1.08464477
R12=1.08676389
R13=1.09081788
R14=1.08424777
R15=1.08705415
A1=124.79924427
A2=118.66531659
A3=118.59818696
A4=123.87936229
A5=124.13037719
A6=117.34889212
A7=124.26579247
A8=118.20372663
A9=116.07062909
A10=119.74894887
A11=121.58695174
A12=118.97168539
A13=121.99553192
A14=115.29338711
D1=0.
D2=0.
D3=180.
D4=180.
D5=0.
D6=0.
D7=0.
D8=180.
D9=180.
D10=180.
D11=0.
D12=180.
D13=180.
|
R1=1.31260842
R2=1.41435513
R3=1.36889083
R4=1.41055187
R5=1.41594546
R6=1.31076411
R7=1.35743362
R8=1.37094537
R9=1.4188397
R10=1.08893784
R11=1.08399806
R12=1.08598547
R13=1.09017221
R14=1.08363495
R15=1.08649156
A1=124.78831373
A2=118.7010846
A3=118.56131879
A4=123.79500007
A5=124.11850196
A6=117.29962815
A7=124.3096893
A8=118.25314352
A9=116.0162935
A10=119.69172932
A11=121.58122421
A12=118.98986424
A13=121.83827904
A14=115.20463797
D1=0.
D2=0.
D3=180.
D4=180.
D5=0.
D6=0.
D7=0.
D8=180.
D9=180.
D10=180.
D11=0.
D12=180.
D13=180.
|
|
|
|
|
|
|
|