|
| |
|
|
| Table 2. 1-Pyrroline molecular structure parameters, ropt(1) = MP2/6-311+G(2d,2p) optimization and ropt(2) = MP2/aug-cc-pVTZ optimization (Å
and degrees). |
| |
|
|
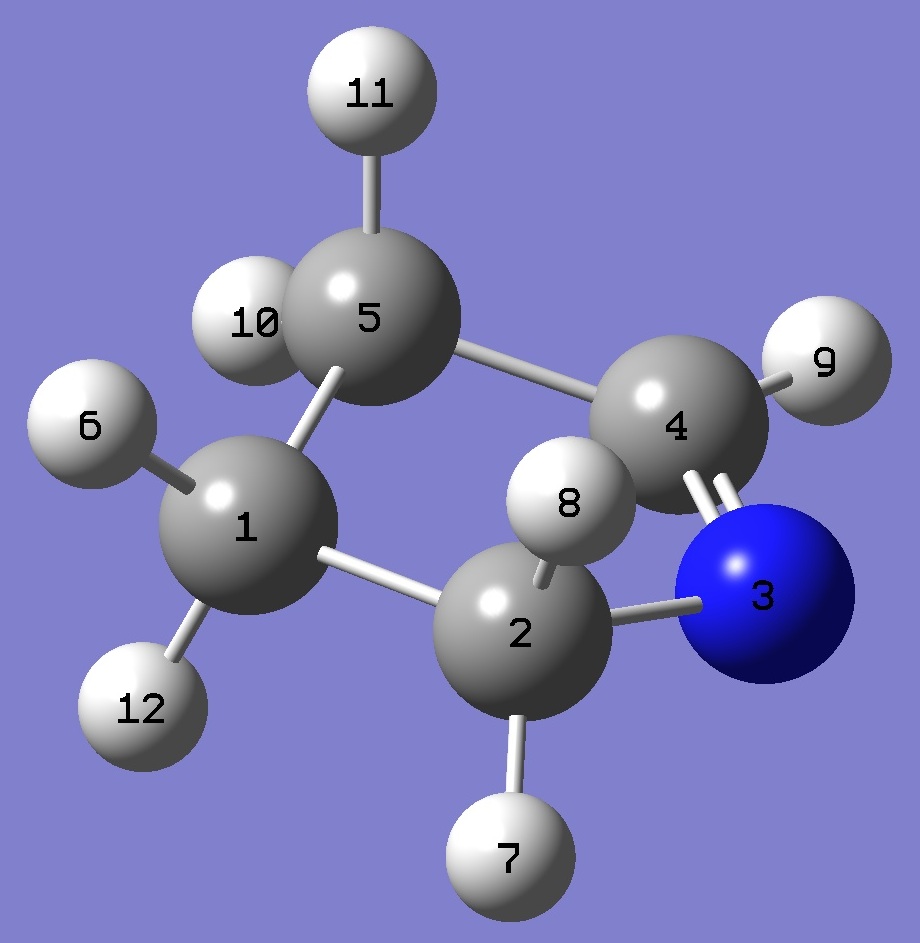
|
C
C,1,B1
N,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
C,4,B4,3,A3,2,D2,0
H,1,B5,2,A4,3,D3,0
H,2,B6,1,A5,5,D4,0
H,2,B7,1,A6,5,D5,0
H,4,B8,3,A7,2,D6,0
H,5,B9,4,A8,3,D7,0
H,5,B10,4,A9,3,D8,0
H,1,B11,2,A10,3,D9,0
|
|
|
| ropt(1) |
ropt(2) |
|
|
|
B1=1.5397481
B2=1.47894139
B3=1.28123894
B4=1.51051369
B5=1.08552817
B6=1.08585939
B7=1.09015021
B8=1.08395024
B9=1.0870939
B10=1.09150085
B11=1.08745494
A1=106.43758491
A2=107.7721161
A3=116.36966484
A4=113.01563843
A5=113.47269772
A6=112.13489423
A7=120.03786504
A8=112.86727336
A9=109.47535158
A10=109.69625567
D1=15.34698044
D2=-0.19242658
D3=-146.04629055
D4=-144.04729095
D5=93.75069535
D6=177.70345543
D7=-136.61145026
D8=103.80996916
D9=93.08546015
|
B1=1.53768936
B2=1.47515699
B3=1.28091379
B4=1.50735347
B5=1.08778683
B6=1.08837722
B7=1.09291486
B8=1.08542848
B9=1.0892125
B10=1.09398507
B11=1.08984022
A1=106.46858466
A2=107.67816308
A3=116.36448951
A4=113.02340285
A5=113.49596145
A6=112.06424776
A7=120.06252446
A8=112.89055124
A9=109.38117858
A10=109.66832463
D1=15.70665011
D2=-0.31655648
D3=-146.46997085
D4=-144.57848819
D5=93.28044781
D6=177.72286047
D7=-136.91453336
D8=103.54102108
D9=92.56411946
|
|
|
|
|
|