| |
||||||||
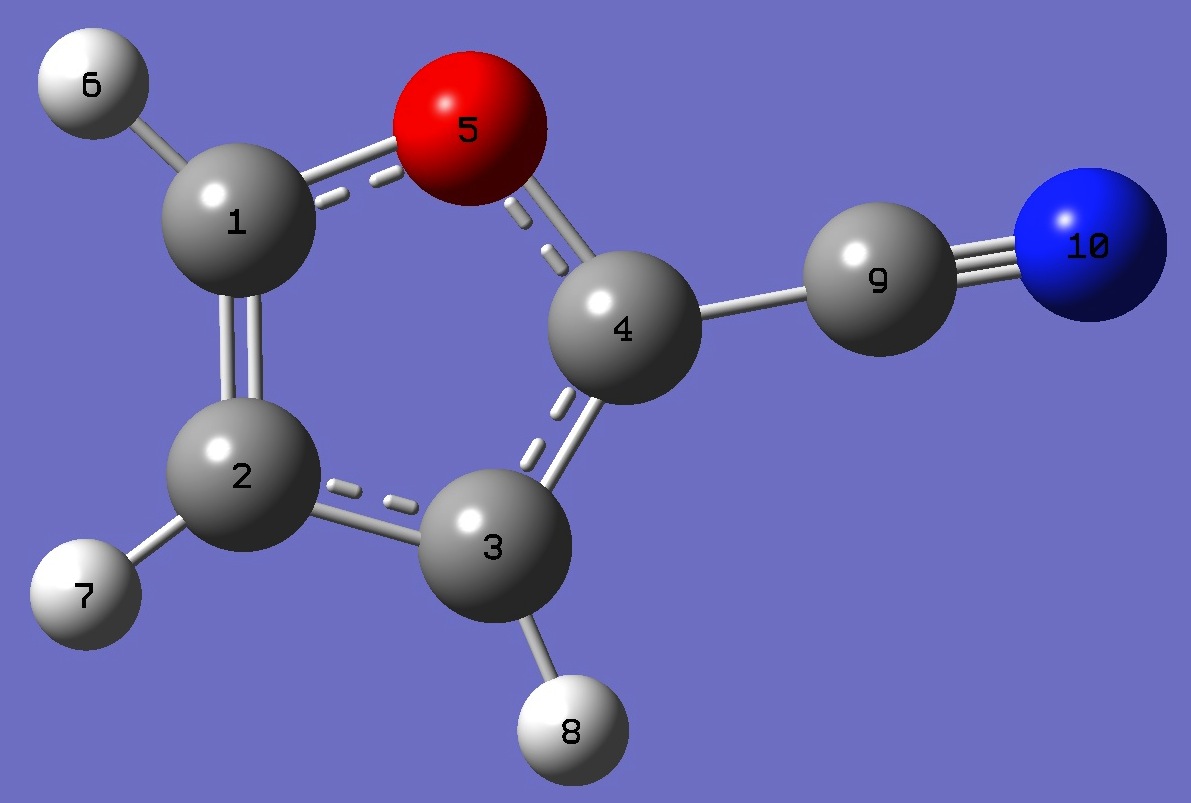
| Table 1. 14N nqcc's in 2-Cyanofuran (MHz). | ||||||||
| Calculation was made on | ||||||||
| the [a] B3P86/6-31G(3d,3p) opt structure. | ||||||||
| the [b] MP2/6-311+G(3df,3pd) opt structure with corrected CN bond length. |
||||||||
| |
||||||||
| Calc. [a] |
Calc. [b] | Expt. [1] |
||||||
| |
||||||||
| Xaa | - |
4.293 |
- |
4.295 |
- |
4.26(5) * |
||
| Xbb | 2.795 |
2.779 |
2.61 * |
|||||
| Xcc | 1.498 |
1.517 |
1.65 * |
|||||
| |Xab| | 0.248 |
0.258 |
||||||
| |
||||||||
| RMS |
0.14 (4.9 %) |
0.13 (4.4 %) |
||||||
| RSD | 0.030 (1.3 %) |
0.030 (1.3 %) | ||||||
| Xxx | 2.804 |
2.788 |
||||||
| Xyy | 1.498 | 1.517 | ||||||
| Xzz | - |
4.302 |
- |
4.305 |
||||
| ETA | - |
0.304 |
- |
0.295 |
||||
| Øz,a | 2.00 |
2.08 |
||||||
| Øa,CN | 1.88 |
1.93 |
||||||
| Øz,CN | 0.12 |
0.15 |
||||||
| Table 3. 2-Chlorothiophene. Rotational Constants (MHz). | ||||
| [a] B3P86/6-31G(3d,3p) opt structure. | ||||
| [b] MP2/6-311+G(3df,3pd) opt structure with corrected CN bond length. | ||||
| Calc [a] | Calc [b] | Expt. [1] | ||
| A |
9285. |
9253. |
9220.106(20) |
|
| B |
2040. |
2038. |
2029.262(11) |
|
| C |
1673. |
1670. |
1662.640(9) |
|