|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH2·NH·CH(CH3) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in 2-Methylaziridine (Propyleneimine)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
microwave spectra of cis-2-methylaziridine
was observed and assigned by Li and Durig [1], and by Schmidt and Beeson [2]. 14N nuclear quadrupole coupling constants were determined.
|
|
|
|
|
|
|
|
|
|
|
|
|
Here, for both cis and trans conformers - shown below, calculation of the nitrogen nqcc
tensor was made on molecular structures given by MP2/6-311+G(d,p) and MP2/6-311+G(3df,3pd) optimization. These
calculated nqcc's are compaed with experimental values [1] in Tables 1 and 2. Structure
parameters are given in Z-Matrix format in Table 3, rotational
constants and dipole moments in Table 4, quartic centrifugal distortion constants in Table 5.
|
|
|
|
|
|
|
|
|
|
|
|
|
cis-2-Methylaziridine
|
|
|
trans-2-Methylaziridine
|
|
|
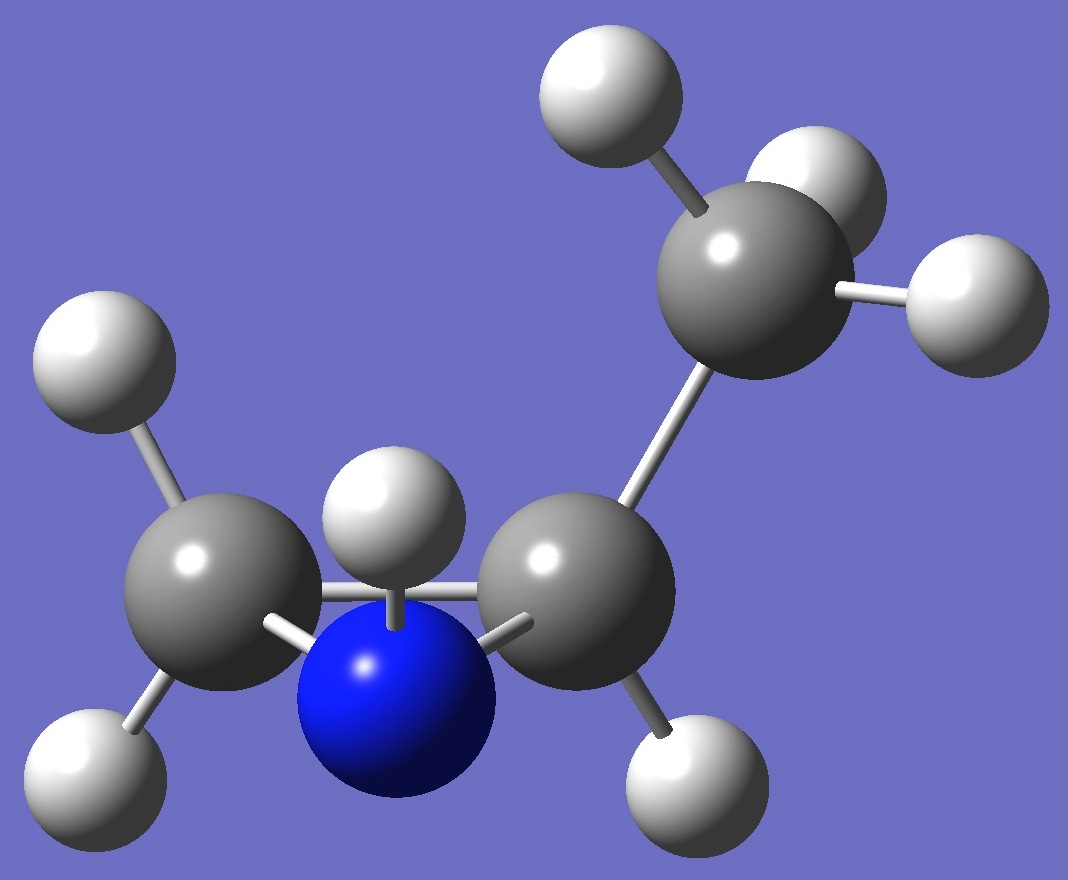
|
At the
|
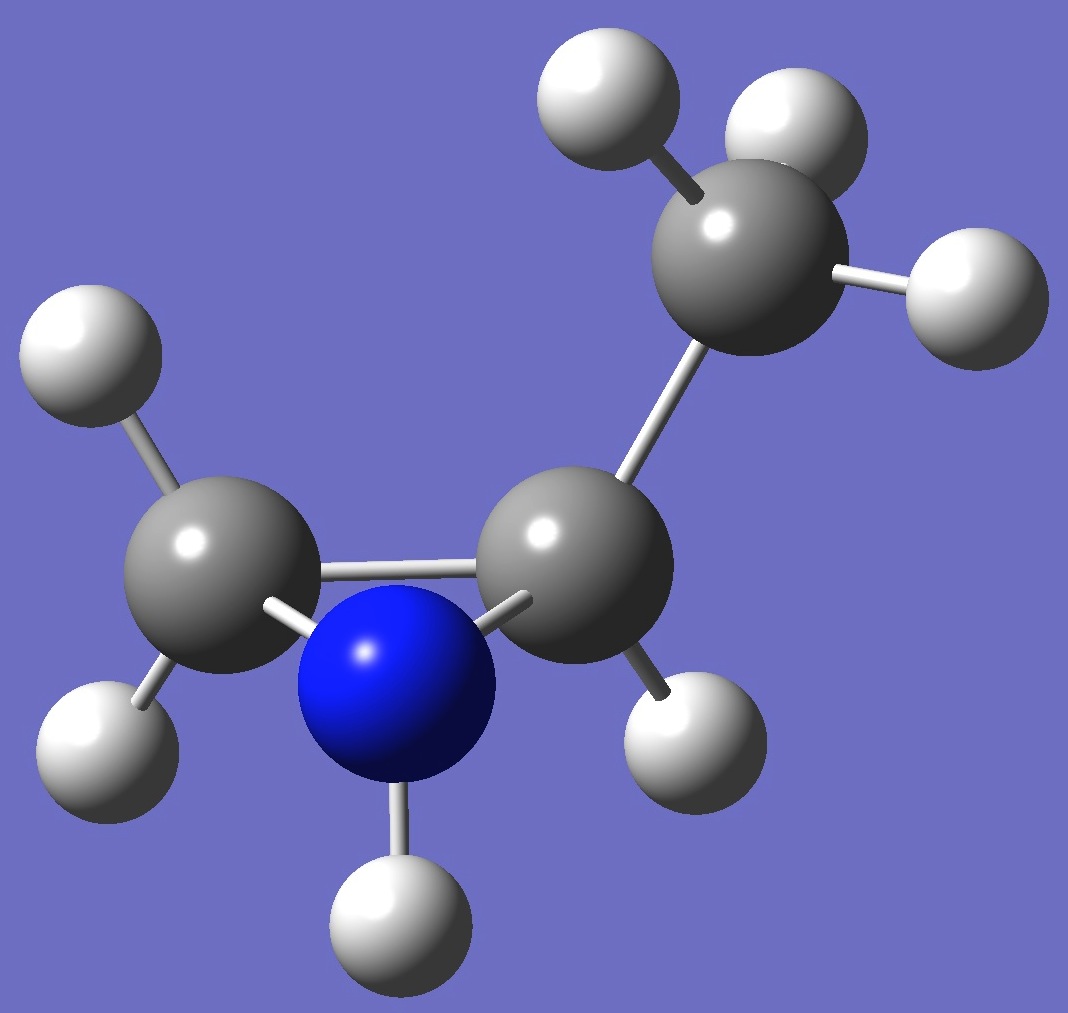
|
|
|
MP2/6-311+G(3df,3pd) |
|
|
level of theory,
|
|
|
Etrans < Ecis
|
|
|
by 2.1 kJ/mol
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz.
Ø (degrees) is the angle between its subscripted parameters.
|
|
|
RMS is the root mean square
difference between calculated and experimental nqcc's. RSD is the
calibration residual standard deviation of
the B3PW91/6-311+G(df,pd) model for calculation of the nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc tensor in cis-2-Methylaziridine
(MHz). Calculation was made on molecular structures given by (1)
MP2/6-311+G(d,p) and (2) MP2/6-311+G(3df,3pd) optimization. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
1.167
|
-
|
1.192
|
-
|
1.16(2)
|
|
|
Xbb |
|
1.778
|
|
1.800
|
|
1.80(2)
|
|
|
Xcc |
-
|
0.611
|
-
|
0.608
|
-
|
0.64(2)
|
|
|
Xab
|
|
0.676
|
|
0.698
|
|
|
|
|
Xac |
|
1.999
|
|
1.999
|
|
|
|
|
Xbc |
-
|
2.106
|
-
|
2.115
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.021 (1.8 %)
|
|
0.026 (2.2 %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.551
|
|
0.552
|
|
|
|
|
Xyy |
|
3.058
|
|
3.077
|
|
|
|
|
Xzz |
-
|
3.609
|
|
3.629
|
|
|
|
|
ETA |
|
0.694
|
|
0.696
|
|
|
|
|
Øz,NH |
|
132.1
|
|
131.8
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N
nqcc tensor in trans-2-Methylaziridine
(MHz). Calculation was made on molecular structures given by (1)
MP2/6-311+G(d,p) and (2) MP2/6-311+G(3df,3pd) optimization. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2)
|
|
Expt
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
0.813
|
|
0.798
|
|
|
|
|
Xbb |
|
2.063
|
|
2.071
|
|
|
|
|
Xcc |
-
|
2.876
|
-
|
2.869
|
|
|
|
|
Xab
|
-
|
1.391
|
-
|
1.406
|
|
|
|
|
Xac |
-
|
0.944
|
-
|
0.963
|
|
|
|
|
Xbc |
-
|
1.244
|
-
|
1.243
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.526
|
|
0.519
|
|
|
|
|
Xyy |
|
3.018
|
|
3.031
|
|
|
|
|
Xzz |
-
|
3.543
|
-
|
3.550
|
|
|
|
|
ETA |
|
0.703
|
|
0.707
|
|
|
|
|
Øz,NH |
|
132.4
|
|
132.3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3. 2-Methylaziridine: MP2/6-311+G(d,p) and MP2/6-311+G(3df,3pd) optimized structure parameters (Å
and degrees).
|
|
|
|
|
|
|
|
|
|
|
| cis-2-Methylaziridine
|
|
trans-2-Methylaziridine
|
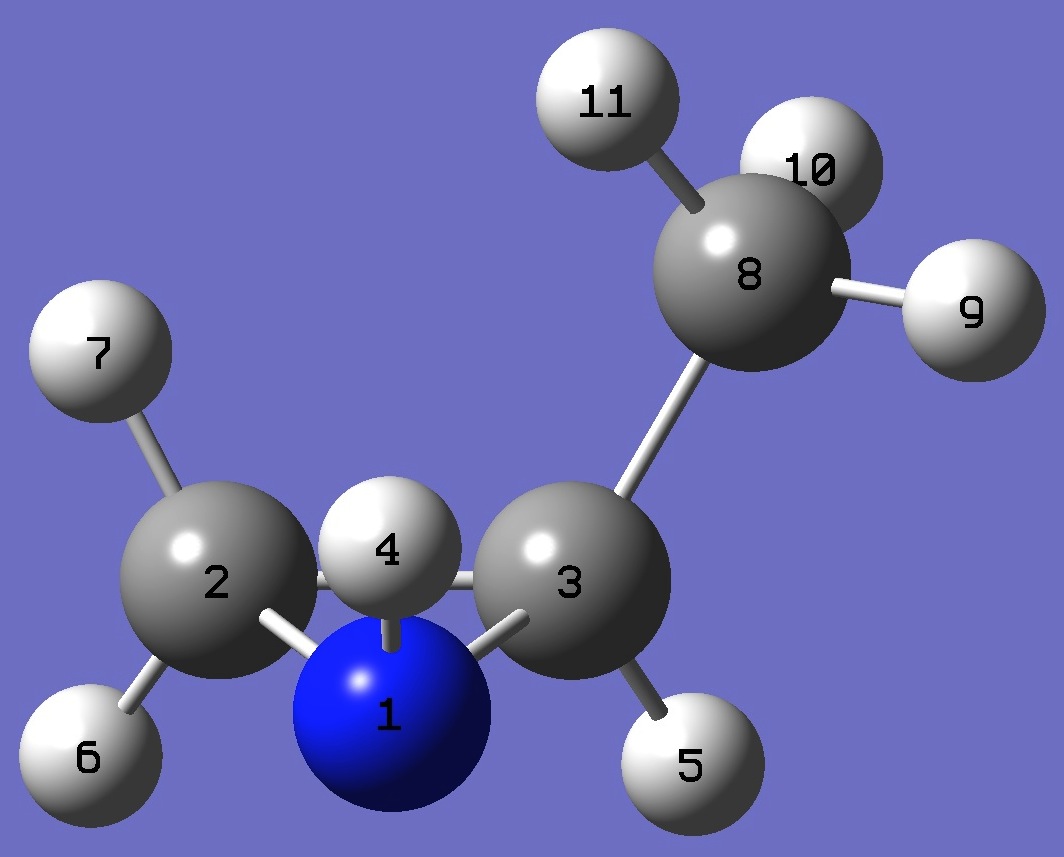
|
N
C,1,B1
C,1,B2,2,A1
H,1,B3,2,A2,3,D1,0
H,3,B4,1,A3,2,D2,0
H,2,B5,1,A4,3,D3,0
H,2,B6,1,A5,3,D4,0
C,3,B7,1,A6,2,D5,0
H,8,B8,3,A7,1,D6,0
H,8,B9,3,A8,1,D7,0
H,8,B10,3,A9,1,D8,0
|
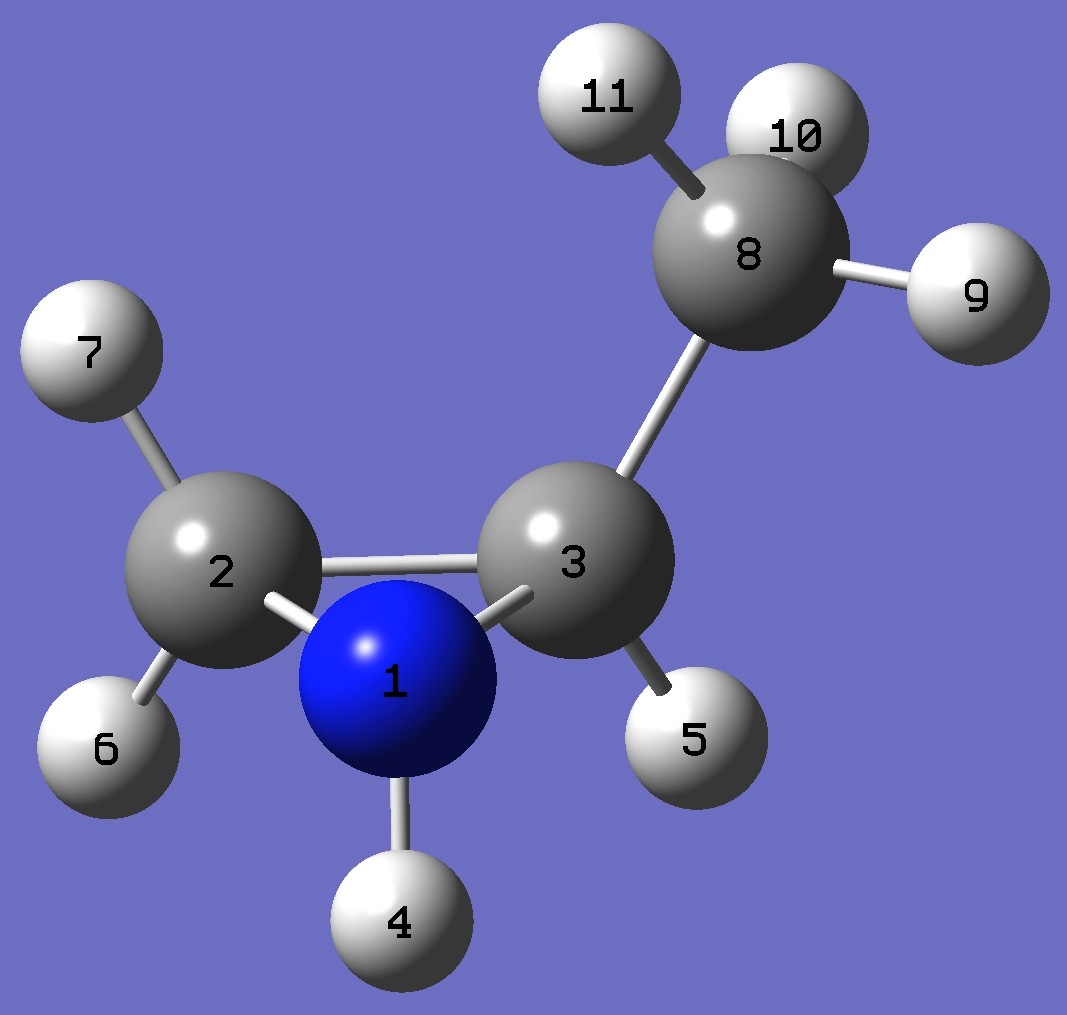
|
|
|
|
|
____________________ MP2/6-311+G(d,p)____________________
|
|
cis
|
|
|
|
|
trans
|
|
|
|
|
B1=1.48455468
B2=1.47718299
B3=1.01960358
B4=1.0869794
B5=1.08404927
B6=1.08672656
B7=1.50764998
B8=1.09465623
B9=1.09467929
B10=1.09562601
A1=60.10403013
A2=108.43032232
A3=112.34906577
A4=114.49349698
A5=118.02587932
A6=119.84653164
A7=110.94814244
A8=110.88386996
A9=110.29693244
D1=101.04559283
D2=-109.62506439
D3=112.1129528
D4=-106.65790104
D5=109.84068261
D6=77.21044473
D7=-162.43218221
D8=-42.82151946
|
|
B1=1.4833335
B2=1.47743414
B3=1.01805204
B4=1.08877059
B5=1.08582525
B6=1.08496902
B7=1.50522401
B8=1.0944112
B9=1.0947942
B10=1.09321847
A1=60.18368895
A2=108.93428396
A3=116.28779387
A4=118.24642232
A5=114.20776719
A6=115.78471968
A7=110.73809611
A8=110.84591935
A9=109.5315166
D1=-102.23126317
D2=-105.94736
D3=108.08212475
D4=-110.7183989
D5=112.85626432
D6=81.42600796
D7=-157.95978902
D8=-37.83352299
|
|
|
|
|
|
|
|
|
|
|
| ____________________ MP2/6-311+G(3df,3pd)____________________ |
|
cis
|
|
|
|
|
trans
|
|
|
|
|
B1=1.47903111
B2=1.47057676
B3=1.01571814
B4=1.08280119
B5=1.07978458
B6=1.0819464
B7=1.50131819
B8=1.0898696
B9=1.0897449
B10=1.09076623
A1=60.09632531
A2=108.67131248
A3=112.33603282
A4=114.30819877
A5=117.87752209
A6=119.47904473
A7=110.96781997
A8=110.998557
A9=110.34458921
D1=101.08504192
D2=-109.60061196
D3=112.20234954
D4=-106.71903851
D5=109.96800372
D6=76.89032242
D7=-162.87302226
D8=-43.11520196
|
|
B1=1.47784688
B2=1.47215681
B3=1.01424742
B4=1.08387826
B5=1.08094524
B6=1.08065069
B7=1.4996737
B8=1.08918986
B9=1.08974834
B10=1.08840126
A1=60.11424879
A2=109.07451674
A3=116.32943696
A4=118.17126295
A5=114.06672114
A6=115.47405408
A7=110.76970213
A8=110.94088848
A9=109.63441925
D1=-102.37611586
D2=-106.06808881
D3=108.31965798
D4=-110.63752867
D5=112.79938152
D6=81.33047717
D7=-158.08515319
D8=-37.89761827
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 4. 2-Methylaziridine: Rotational Constants (MHz) and Dipole Moments (D). Calc (1)
= MP2/6-311+G(d,p) and Calc (2) = MP2/6-311+G(3df,3pd) optimization.
|
|
|
|
|
|
|
|
|
__________cis-2-Methylaziridine__________
|
|
|
|
Calc (1)
|
Calc (2)
|
Expt [1]
|
|
|
|
|
|
|
|
|
A |
16689
|
16813 |
|
|
|
B |
6561
|
6624 |
|
|
|
C |
5818
|
5875 |
|
|
|
|µa|
|
1.41
|
1.40
|
|
|
|
|µb| |
1.10
|
1.10
|
|
|
|
|µc| |
0.87
|
0.87
|
|
|
|
|
|
|
|
|
|
|
__________trans-2-Methylaziridine__________ |
|
|
|
Calc (1)
|
Calc (2)
|
Expt [1]
|
|
|
|
|
|
|
|
|
A
|
16928
|
17044
|
|
|
|
B
|
6535
|
6596
|
|
|
|
C
|
5765
|
5821
|
|
|
|
|µa| |
0.11
|
0.12
|
|
|
|
|µb| |
0.88
|
0.88
|
|
|
|
|µc|
|
1.54
|
1.54
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 5. 2-Methylaziridine: Quartic Centrifugal Distortion Constants (kHz). Calc
= B3LYP/cc-pVTZ |
|
|
|
|
|
|
|
|
|
____cis-2-Methylaziridine____
|
|
___trans-2-Methylaziridine___ |
|
|
Calc
|
Expt
|
|
Calc
|
Expt
|
|
|
|
|
|
|
|
Delta_J
|
|
2.73
|
|
|
2.72
|
|
| Delta_JK |
|
4.21
|
|
|
3.21
|
|
| Delta_K |
|
12.8
|
|
|
15.5
|
|
| delta_J |
|
0.188
|
|
|
0.241
|
|
| delta_K |
|
3.25
|
|
|
3.48
|
|
|
|
|
|
|
|
|
| D_J |
|
2.68
|
|
|
2.66
|
|
| D_JK |
|
4.55
|
|
|
3.57
|
|
| D_K |
|
12.5
|
|
|
15.2
|
|
d_1
|
-
|
0.188
|
|
-
|
0.241
|
|
d_2
|
-
|
0.0281
|
|
-
|
00303
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] Y.S.Li and J.R.Durig, J. Mol. Spectrosc. 47,179(1973).
|
|
|
[2] R.Schmidt and E.L.Beeson Jr., J.Chem.Phys. 59,3070(1973): Xaa, Xbb, Xcc = -1.14(6), 2.01(5), -0.88(7) MHz.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Aziridine
|
2-Cyanoaziridine
|
1-Aziridineethanol
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2MeAziridine.html |
|
|
|
|
|
|
Last
Modified 30 Jan 2014
|
|
|
|
|
|
|
|
|
|
|



