|
| |
|
|
|
|
|
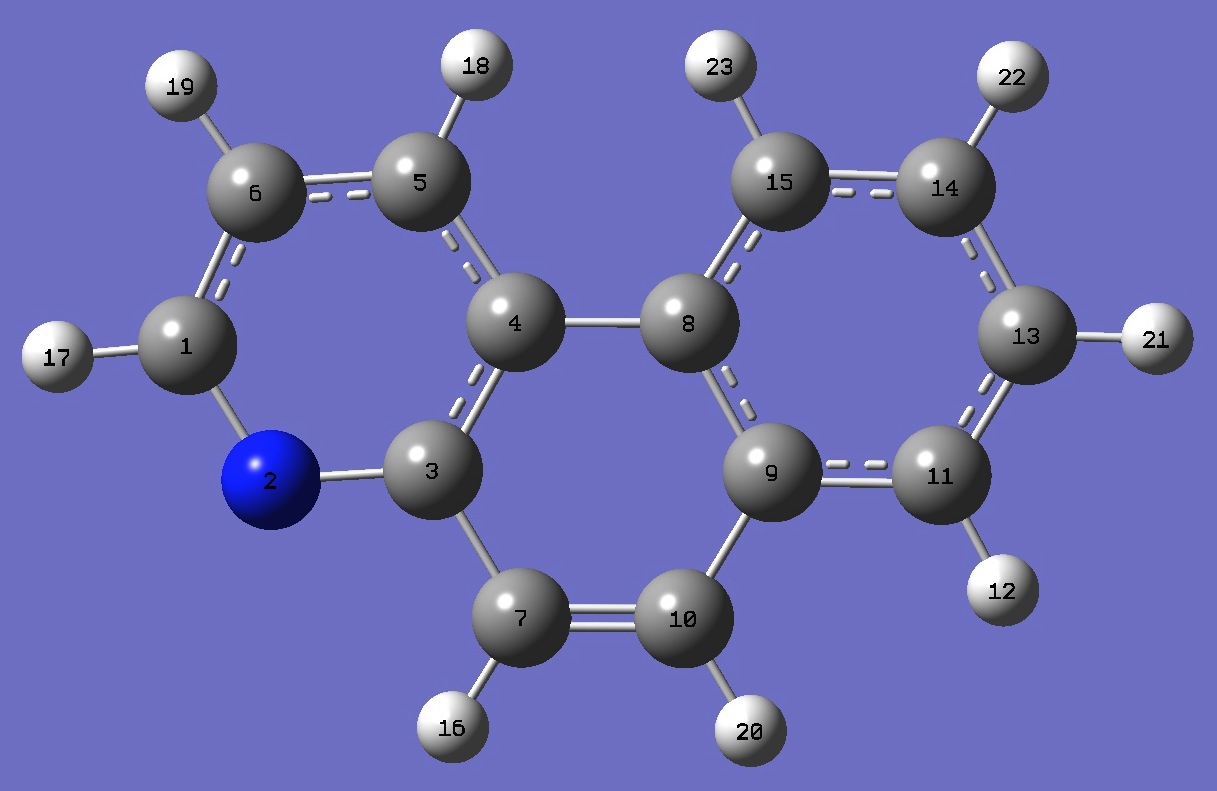
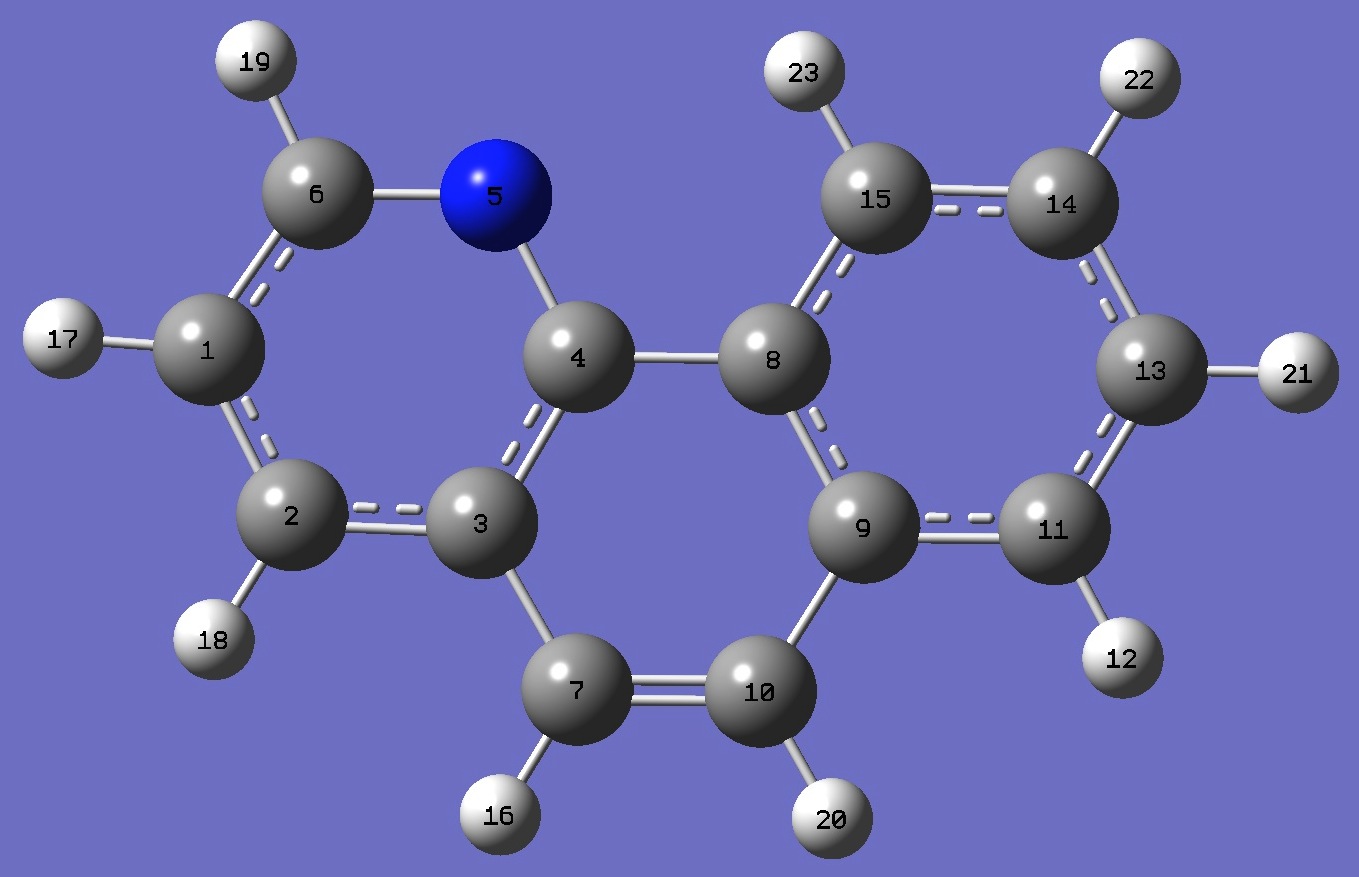
| Table 3. Benzoquinoline. B3P86/6-31G(d,p) and B3P86/6-31G(3d,3p) optimized structure parameters
(Å and degrees). |
| |
|
|
|
|
|
|
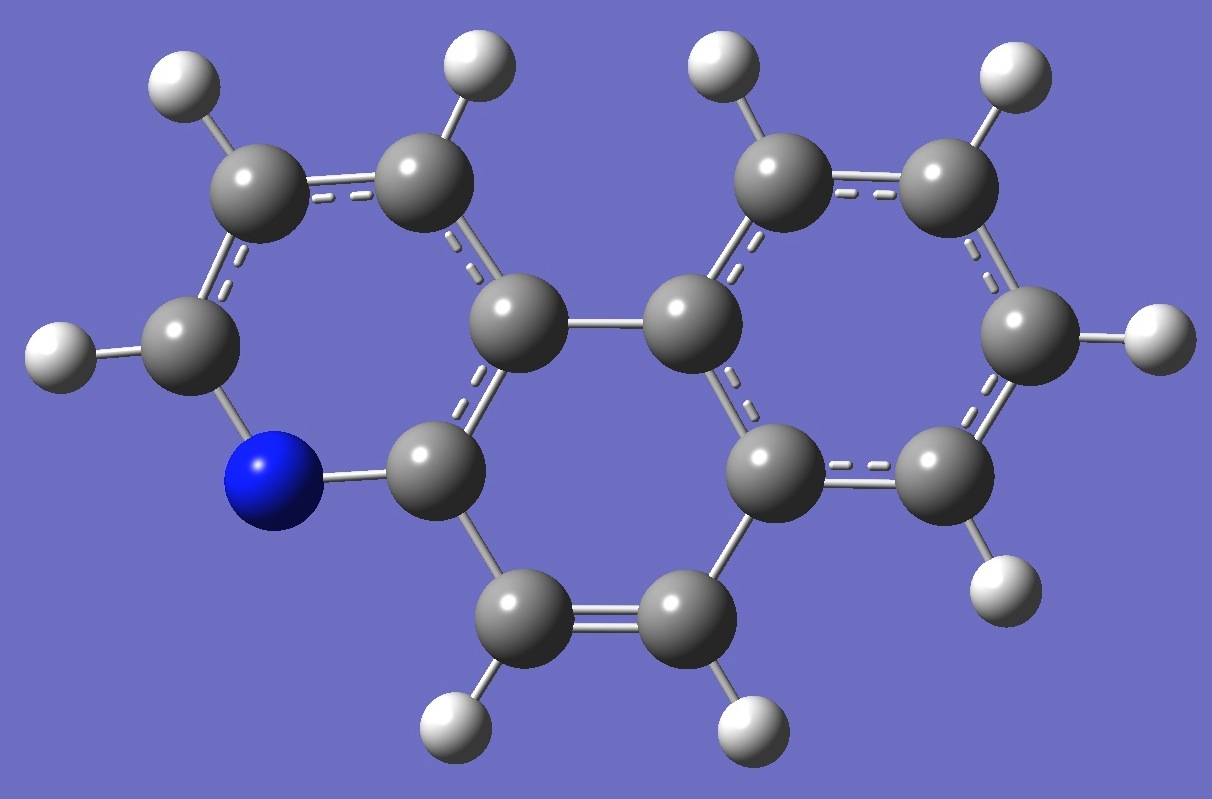
5,6-Benzoquinoline
|
|
|
|
|
|
|
|
|
| C
N,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
C,4,B4,3,A3,2,D2,0
C,5,B5,4,A4,3,D3,0
C,3,B6,2,A5,1,D4,0
C,4,B7,3,A6,2,D5,0
C,8,B8,4,A7,3,D6,0
C,7,B9,3,A8,2,D7,0
C,9,B10,8,A9,4,D8,0
H,11,B11,9,A10,8,D9,0
C,11,B12,9,A11,8,D10,0
C,13,B13,11,A12,9,D11,0
C,14,B14,13,A13,11,D12,0
H,7,B15,3,A14,2,D13,0
H,1,B16,2,A15,3,D14,0
H,5,B17,4,A16,3,D15,0
H,6,B18,5,A17,4,D16,0
H,10,B19,7,A18,3,D17,0
H,13,B20,11,A19,9,D18,0
H,14,B21,13,A20,11,D19,0
H,15,B22,14,A21,13,D20,0
|
|
|
|
|
|
|
|
|
|
6-31G(d,p)
|
6-31G(3d,3p)
|
|
|
|
|
|
|
|
|
|
B1=1.31952357
B2=1.35436084
B3=1.421549
B4=1.40770048
B5=1.37786988
B6=1.43066893
B7=1.44950861
B8=1.42200586
B9=1.35733935
B10=1.41042002
B11=1.08662098
B12=1.37772582
B13=1.4040045
B14=1.3797123
B15=1.08459718
B16=1.08902805
B17=1.08482449
B18=1.0848428
B19=1.08664783
B20=1.08547748
B21=1.08563545
B22=1.0846603
A1=117.91436995
A2=123.24161926
A3=116.66516815
A4=119.86027325
A5=117.00172395
A6=119.50301953
A7=118.82131696
A8=120.7694954
A9=119.4920592
A10=118.55857548
A11=121.01907326
A12=119.64891097
A13=120.3670719
A14=117.01292706
A15=116.29098034
A16=120.50117501
A17=121.12172484
A18=120.46014829
A19=120.31274441
A20=119.888598
A21=118.81641286
D1=0.
D2=0.
D3=0.
D4=180.
D5=180.
D6=0.
D7=180.
D8=180.
D9=180.
D10=0.
D11=0.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
D17=180.
D18=180.
D19=180.
D20=180.
|
B1=1.31725937
B2=1.35330521
B3=1.41909687
B4=1.40518559
B5=1.3754373
B6=1.42948195
B7=1.4477854
B8=1.41961085
B9=1.35512712
B10=1.40864192
B11=1.08583667
B12=1.37573791
B13=1.40198456
B14=1.37769169
B15=1.08403092
B16=1.08841435
B17=1.08394328
B18=1.08422203
B19=1.08592735
B20=1.08470644
B21=1.08484006
B22=1.08377478
A1=117.889443
A2=123.22171811
A3=116.71340028
A4=119.81294159
A5=117.03007977
A6=119.51416648
A7=118.83560116
A8=120.748826
A9=119.5007657
A10=118.60218093
A11=120.99529394
A12=119.66239232
A13=120.36754244
A14=117.12552376
A15=116.264773
A16=120.52469453
A17=121.13286566
A18=120.44840093
A19=120.29740897
A20=119.89443901
A21=118.82261299
D1=0.
D2=0.
D3=0.
D4=180.
D5=180.
D6=0.
D7=180.
D8=180.
D9=180.
D10=0.
D11=0.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
D17=180.
D18=180.
D19=180.
D20=180.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
7,8-Benzoquinoline |
|
|
|
|
|
|
|
|
| C
C,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
N,4,B4,3,A3,2,D2,0
C,5,B5,4,A4,3,D3,0
C,3,B6,2,A5,1,D4,0
C,4,B7,3,A6,2,D5,0
C,8,B8,4,A7,3,D6,0
C,7,B9,3,A8,2,D7,0
C,9,B10,8,A9,4,D8,0
H,11,B11,9,A10,8,D9,0
C,11,B12,9,A11,8,D10,0
C,13,B13,11,A12,9,D11,0
C,14,B14,13,A13,11,D12,0
H,7,B15,3,A14,2,D13,0
H,1,B16,2,A15,3,D14,0
H,2,B17,1,A16,6,D15,0
H,6,B18,5,A17,4,D16,0
H,10,B19,7,A18,3,D17,0
H,13,B20,11,A19,9,D18,0
H,14,B21,13,A20,11,D19,0
H,15,B22,14,A21,13,D20,0
|
|
|
|
|
|
|
|
|
|
6-31G(d,p)
|
6-31G(3d,3p)
|
|
|
|
|
|
|
|
|
|
B1=1.37703471
B2=1.40760874
B3=1.42005605
B4=1.35021018
B5=1.32056601
B6=1.43011748
B7=1.44745249
B8=1.41951301
B9=1.35883573
B10=1.41051426
B11=1.08670194
B12=1.37872236
B13=1.40565872
B14=1.37948628
B15=1.08666637
B16=1.08481541
B17=1.08700894
B18=1.08885902
B19=1.08662229
B20=1.08580295
B21=1.08575467
B22=1.08421953
A1=119.52866212
A2=117.61175185
A3=122.36019388
A4=118.29110943
A5=122.58381288
A6=119.34075437
A7=119.16518394
A8=120.78406724
A9=118.97144936
A10=118.84080799
A11=120.72486724
A12=120.09345369
A13=120.32786267
A14=118.53124071
A15=121.50652436
A16=121.07178831
A17=116.20842351
A18=120.374636
A19=120.01232779
A20=119.76827475
A21=121.61876794
D1=0.
D2=0.
D3=0.
D4=180.
D5=180.
D6=0.
D7=180.
D8=180.
D9=180.
D10=0.
D11=0.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
D17=180.
D18=180.
D19=180.
D20=180.
|
B1=1.37469763
B2=1.40547107
B3=1.41747957
B4=1.34910844
B5=1.31823076
B6=1.4288811
B7=1.44626289
B8=1.41725626
B9=1.35657492
B10=1.40888729
B11=1.08586267
B12=1.37652398
B13=1.40365788
B14=1.37737216
B15=1.08591572
B16=1.08411053
B17=1.08620864
B18=1.08841736
B19=1.08582758
B20=1.08501219
B21=1.08498739
B22=1.08345662
A1=119.48548598
A2=117.65652406
A3=122.34027909
A4=118.25954478
A5=122.49810868
A6=119.30263622
A7=119.16130081
A8=120.78300256
A9=119.01094372
A10=118.85739366
A11=120.71471793
A12=120.09073686
A13=120.33148041
A14=118.52966222
A15=121.53604549
A16=121.06782612
A17=116.15571438
A18=120.38779389
A19=120.02693946
A20=119.75477516
A21=121.51311785
D1=0.
D2=0.
D3=0.
D4=180.
D5=180.
D6=0.
D7=180.
D8=180.
D9=180.
D10=0.
D11=0.
D12=0.
D13=0.
D14=180.
D15=180.
D16=180.
D17=180.
D18=180.
D19=180.
D20=180.
|
|
|
|
|
|
|
|
|
|