| |
||||||||
| Table
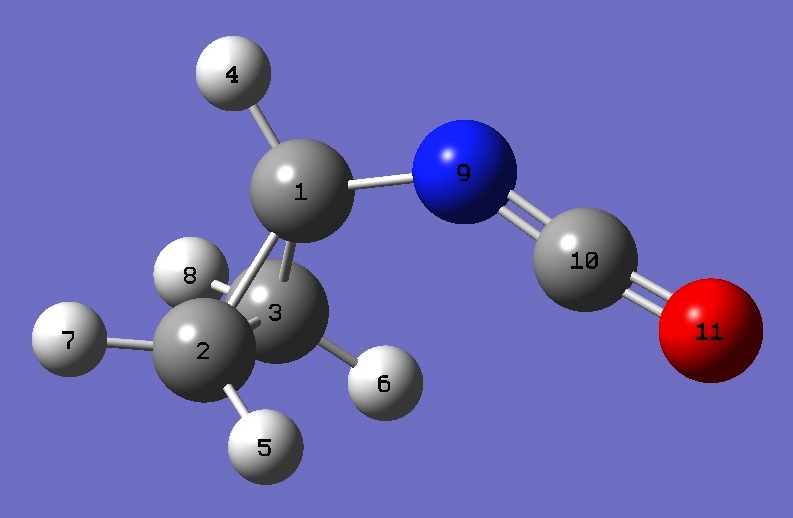
1. 14N nqcc's in cis Cyclopropyl Isocyanate
(MHz). Calculation was made on B3LYP/6-311+G(3df,3pd) and MP2/6-311+G(3df,3pd) optimized molecular structures. |
||||||||
| |
||||||||
| Calc /B3LYP |
Calc /MP2 |
Expt. [1] |
||||||
| |
||||||||
| Xaa | 2.603 |
2.534 |
2.5647(49) |
|||||
| Xbb | - |
1.130 |
- |
1.030 |
- |
1.0624(75) |
||
| Xcc | - |
1.474 |
- |
1.504 |
- |
1.5022(75) |
||
| |Xab| | 1.064 |
1.152 |
||||||
| |
||||||||
| RMS |
0.048 (2.8 %) |
0.026 (1.5 %) |
||||||
| RSD | 0.030 (1.3 %) |
0.030 (1.3 %) | ||||||
| Xxx | - |
1.411 |
- |
1.370 |
||||
| Xyy | - |
1.474 |
- |
1.504 |
||||
| Xzz | 2.885 |
2.874 |
||||||
| ETA | 0.0215 |
0.0467 |
||||||
| Øz,a | 14.84 |
16.44 |
||||||
| Øa,N=C | 33.39 |
35.90 |
||||||
| Øz,N=C | 18.55 |
19.46 |
||||||
| |
||||||||
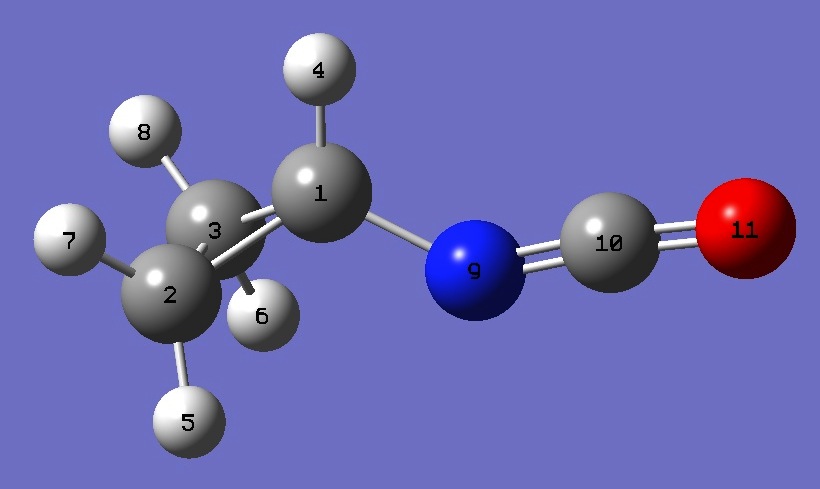
| Table 2. 14N nqcc's in trans Cyclopropyl Isocyanate
(MHz). Calculation was made on B3LYP/6-311+G(3df,3pd) and MP2/6-311+G(3df,3pd) optimized molecular structures. |
||||||||
| |
||||||||
| Calc /B3LYP |
Calc /MP2 |
Expt. [1] |
||||||
| |
||||||||
| Xaa | 2.668 |
2.672 |
2.6306(26) |
|||||
| Xbb | - |
1.393 |
- |
1.422 |
- |
1.3839(31) |
||
| Xcc | - |
1.275 |
- |
1.250 |
- |
1.2467(31) |
||
| |Xac| | 0.797 |
0.725 |
||||||
| |
||||||||
| RMS |
0.028 (1.6 %) |
0.032 (1.8 %) |
||||||
| RSD | 0.030 (1.3 %) |
0.030 (1.3 %) | ||||||
| Xxx | - |
1.430 |
- |
1.380 |
||||
| Xyy | - |
1.393 |
- |
1.422 |
||||
| Xzz | 2.823 |
2.882 |
||||||
| ETA | - |
0.0130 |
0.0148 |
|||||
| Øz,a | 11.01 |
10.15 |
||||||
| Øa,N=C | 10.19 |
11.10 |
||||||
| Øz,N=C | 21.20 |
21.25 |
||||||
| |
||||
| Table 4. Cyclopropyl Isocyanate. Rotational Constants (MHz). Calc = B3LYP/6-311+G(3df,3pd) and MP2/6-311+G(3df,3pd) optimized molecular structures. | ||||
| Calc /B3LYP | Calc /MP2 | Expt. [1] | ||
| cis | A |
10806. |
10304. |
10235.(3) |
| B |
2089. |
2179. |
2186.556(3) |
|
| C |
2034. |
2098. |
2106.168(3) |
|
| trans | A |
17300. |
17226. |
16941.865(3) |
| B |
1764. |
1772. |
1784.3117(2) |
|
| C |
1700. |
1708. |
1716.1281(3) |
|