|
|
|
|
Table 3. Isopropylamine: MP2/aug-cc-pVDZ and MP2/aug-cc-pVTZ ropt structure parameters (Å and degrees).
|
|
|
|
|
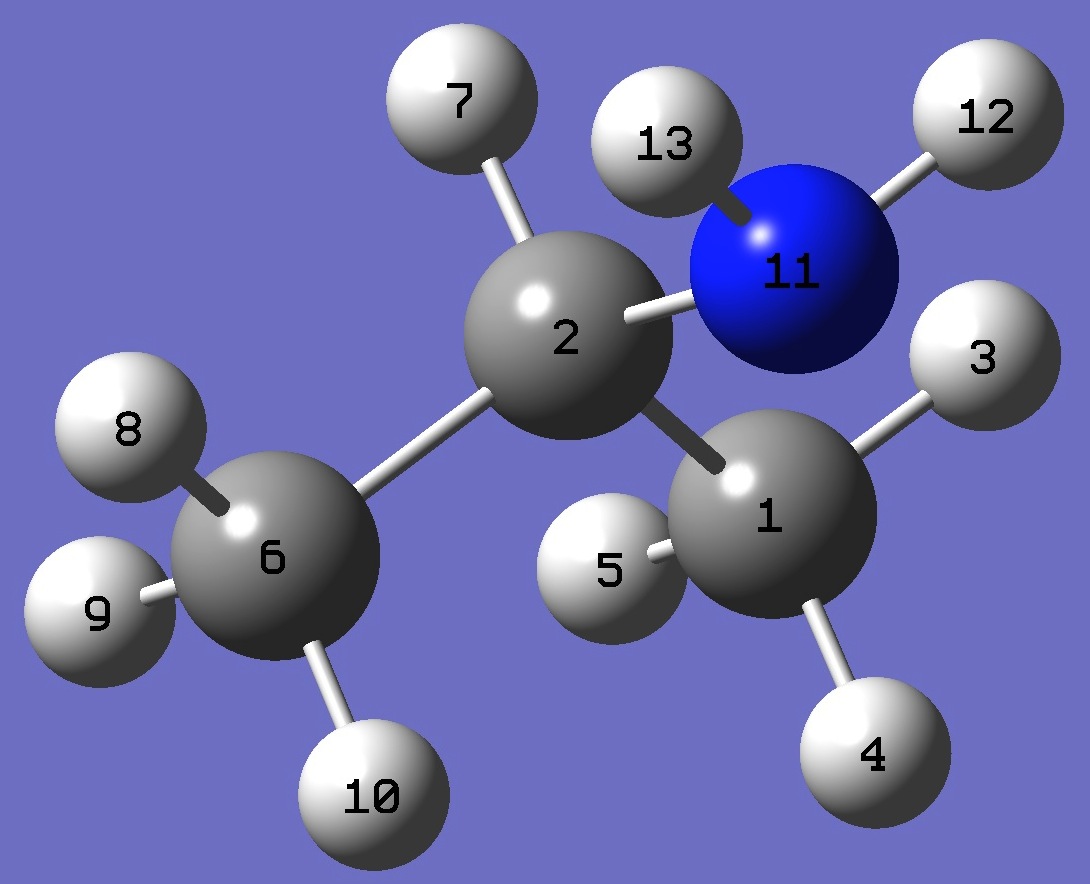
C
C,1,B1
H,1,B2,2,A1
H,1,B3,2,A2,3,D1,0
H,1,B4,2,A3,3,D2,0
C,2,B5,1,A4,3,D3,0
H,2,B6,1,A5,6,D4,0
H,6,B7,2,A6,1,D5,0
H,6,B8,2,A7,1,D6,0
H,6,B9,2,A8,1,D7,0
N,2,B10,1,A9,6,D8,0
H,11,B11,2,A10,1,D9,0
H,11,B12,2,A11,1,D10,0
|
|
|
| aug-cc-pVDZ
| aug-cc-pVTZ |
|
|
|
|
B1=1.52899126
B2=1.10275494
B3=1.10177236
B4=1.10173987
B5=1.52899126
B6=1.10993146
B7=1.10275494
B8=1.10173987
B9=1.10177236
B10=1.4756914
B11=1.02168445
B12=1.02168445
A1=111.04758362
A2=109.75632427
A3=110.7785287
A4=111.27722882
A5=108.3182296
A6=111.04758362
A7=110.7785287
A8=109.75632427
A9=108.37019636
A10=110.12883353
A11=110.12883353
D1=119.6383508
D2=-119.90758292
D3=-179.29574219
D4=-118.96120407
D5=179.29574219
D6=-60.79667489
D7=59.65739139
D8=119.05766799
D9=61.2332996
D10=177.89123452
|
B1=1.51927321
B2=1.09113044
B3=1.09017195
B4=1.0899314
B5=1.51927321
B6=1.09830904
B7=1.09113044
B8=1.0899314
B9=1.09017195
B10=1.46639852
B11=1.01419573
B12=1.01419573
A1=111.03684156
A2=109.66327716
A3=110.79694653
A4=111.16145245
A5=108.32231279
A6=111.03684156
A7=110.79694653
A8=109.66327716
A9=108.44917943
A10=110.38704369
A11=110.38704369
D1=119.58054683
D2=-120.0764514
D3=-179.21333594
D4=-118.90012047
D5=179.21333594
D6=-60.71021267
D7=59.63278911
D8=119.13524043
D9=60.77613428
D10=178.39539191
|
|
|