|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
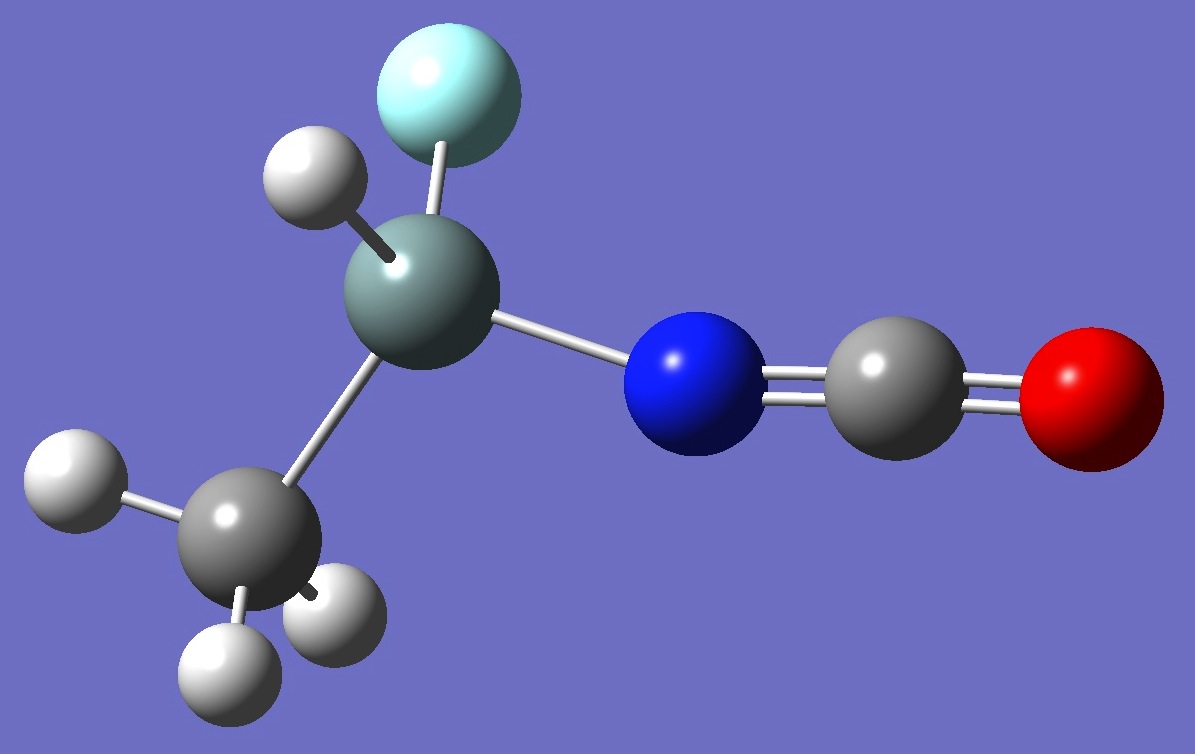
CH3-SiHF-N=C=O
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Methylfluoroisocyanate Silane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The microwave
spectrum of methylfluoroisocyanate silane was observed and assigned by Seifert, Guirgis, et al.
[1,2].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 14N nqcc tensor was made here on ropt
molecular structures given by HF/6-31G(3d,3p) and HF/6-311G(3d,3p)
optimization, and on a MP3/aug-cc-pVTZ optimized structure derived by
Guirgis et al. [2]. These calculated nqcc's are compared with the
experimental values [1,2] in Table 1. Structure
parameters in Z-matrix format for the HF optimizations are
compared in Table 2, rotational
constants and dipole moments in Table 3, and centrifugal distortion
constants in Table 4. For the MP3 optimization, see Ref. [2].
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø is the angle between its subcripted parameters.
|
|
|
RMS is root mean square difference between calculated and experimental nqcc's. RSD is the
calibration residual standard deviation of the B3PW91/6-311+G(df,pd) model for calculation of nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Table 1. 14N nqcc's in CH3-SiHF-N=C=O (MHz). Calculation was made on (1) HF/6-31G(3d,3p), (2) HF/6-311G(3d,3p), and (3) MP3/aug-cc-pVTZ ropt structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Calc (3) |
|
Expt [1,2] *
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Xaa |
|
1.726
|
|
1.737
|
|
1.811
|
|
1.770(7)
|
|
|
Xbb |
-
|
0.855
|
-
|
0.857
|
-
|
0.891
|
-
|
0.839(36)
|
|
|
Xcc |
-
|
0.871
|
-
|
0.880
|
-
|
0.919
|
-
|
0.931(36)
|
|
|
Xab |
|
0.139
|
|
0.121
|
|
0.149
|
|
|
|
|
Xac |
-
|
0.408
|
-
|
0.250
|
-
|
0.254
|
|
|
|
|
Xbc |
-
|
0.033
|
-
|
0.004
|
|
0.002
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.044 (3.7 %)
|
|
0.037 (3.1 %)
|
|
0.039 (3.3 %)
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
-
|
0.861
|
-
|
0.858
|
-
|
0.894
|
|
|
|
|
Xyy |
-
|
0.935 |
-
|
0.908
|
-
|
0.948
|
|
|
|
|
Xzz |
|
1.796
|
|
1.766
|
|
1.842
|
|
|
|
|
ETA |
|
0.0413
|
|
0.0285
|
|
0.0291
|
|
|
|
|
Øz,N=C |
|
6.95
|
|
8.33
|
|
7.18
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Xaa, Xbb, Xcc derived here from experimental 1.5Xaa = 2.655(11) and 0.25(Xbb - Xcc) = 0.023(18) MHz.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Table 2. CH3-SiHF-N=C=O. HF/6-31G(3d,3p) and HF/6-311G(3d,3p) ropt structure parameters (Å
and degrees).
|
|
|
|
|
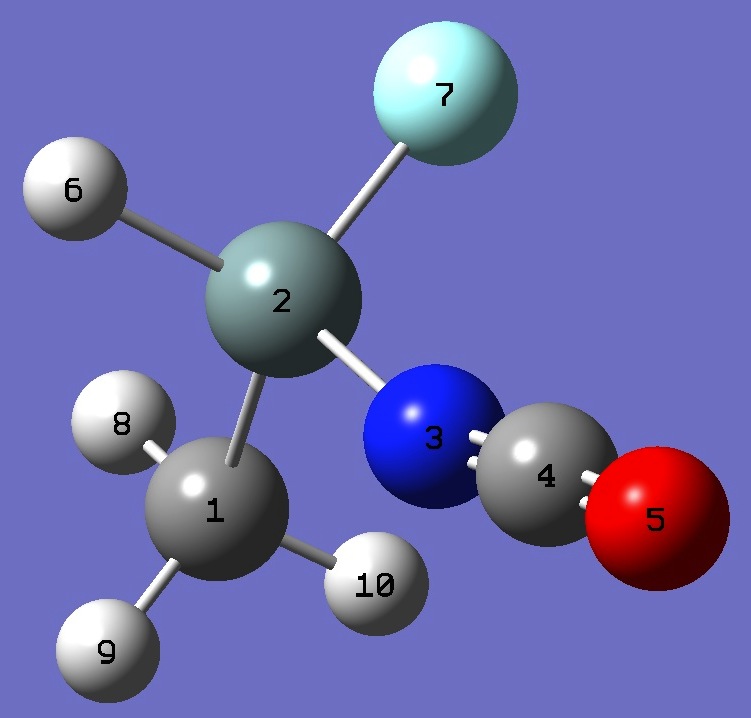
|
C
Si,1,B1
N,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
O,4,B4,3,A3,2,D2,0
H,2,B5,3,A4,4,D3,0
F,2,B6,3,A5,4,D4,0
H,1,B7,2,A6,3,D5,0
H,1,B8,2,A7,3,D6,0
H,1,B9,2,A8,3,D7,0
|
|
|
|
HF/6-31G(3d,3p)
|
HF/6-311G(3d,3p)
|
|
|
|
|
|
|
B1=1.8474159
B2=1.70124874
B3=1.18327343
B4=1.14431429
B5=1.46291508
B6=1.5844807
B7=1.08643881
B8=1.08656174
B9=1.08608598
A1=110.11742251
A2=157.22143802
A3=177.80777346
A4=108.6715672
A5=107.88320335
A6=110.70612224
A7=110.51918747
A8=111.0796777
D1=147.37312784
D2=-179.27358425
D3=-88.36014533
D4=27.88917231
D5=-179.12477079
D6=61.13153467
D7=-58.86481063
|
B1=1.84616011
B2=1.7032079
B3=1.18108404
B4=1.14017693
B5=1.46447886
B6=1.58238689
B7=1.08405763
B8=1.08443362
B9=1.08378141
A1=109.85429306
A2=155.04342237
A3=177.6985679
A4=109.21623096
A5=107.66011147
A6=110.71229712
A7=110.21230887
A8=110.9222252
D1=-174.71678886
D2=176.30460511
D3=-50.07439864
D4=66.02908308
D5=-178.630018
D6=61.63761453
D7=-58.19207679
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Table 3. CH3-SiHF-N=C=O. Rotational Constants (MHz) and Dipole Moments (D). (1) HF/6-31G(3d,3p) and (2) HF/6-311G(3d,3p) ropt structures.
|
|
|
|
|
|
|
|
Calc (1)
|
Calc (2)
| Expt [1]
|
|
|
|
|
|
|
A
|
6163.7
|
6340.3
| 6301.415(45)
|
|
B
|
1565.4
|
1548.0
|
1535.078(39)
|
|
C
|
1342.0
|
1320.3
|
1310.485(39)
|
|
|µa|
|
2.05
|
1.93
|
|
|
|µb| |
1.40
|
1.44
|
|
|
|µc| |
0.52
|
0.61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
Table 4. CH3-SiHF-N=C=O. Quartic Centrifugal Distortion Constants (kHz). Calc = B3LYP/cc-pVTZ
|
|
|
|
|
|
|
|
|
|
Calc
|
|
Expt [1]
|
|
|
|
|
|
|
|
Delta_J
|
|
0.566
|
|
0.742(33)
|
|
Delta_JK |
|
23.7
|
|
41.50(14)
|
|
Delta_K |
|
1.06
|
-
|
25.39(10)
|
|
delta_J |
|
0.0184
|
|
0.067(13)
|
|
delta_K |
|
12.6
|
|
25.6(19)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1]
N.A.Seifert, S.Lobsiger, B.H.Pate, G.A.Guirgis, J.S.Overby, and
J.R.Durig, Abstract RC12, 68th International Symposium on Molecular
Spectroscopy, 2013.
|
|
|
[2] G.A.Guirgis, J.S.Overby, T.J.Barker, M.H.Palmer, B.H.Pate, and N.A.Seifert, J.Phys.Chem. A, 119(4),652(2015).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Methyldifluoroisocyanate Silane
|
CH3CH2N=C=O
|
Acetylisocyanate
|
HNCO
|
|
|
Difluoroisocyanato Silane
|
Vinylisocyanate
|
tert-Butylisocyanate
|
ClNCO
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3SiHFNCO.html |
|
|
|
|
|
|
Last
Modified 9 Dec 2016 |
|
|
|
|
|
|
|
|
|
|

