|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHF2-CH2NH2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in 2,2-Difluoroethylamine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
microwave spectrum of 2,2-difluoroethylamine has been
investigated by Marstokk and Mųllendal [1].
|
|
|
Calculation of the 14N
nuclear quadrupole coupling constant tensor in each of the following rotamers of the title molecule was made here on ropt molecular
structures given by MP2/6-311+G(3df,3pd) and MP2/aug-cc-pVTZ optimization.
|
|
|
|
|
|
|
|
|
|
|
|
|
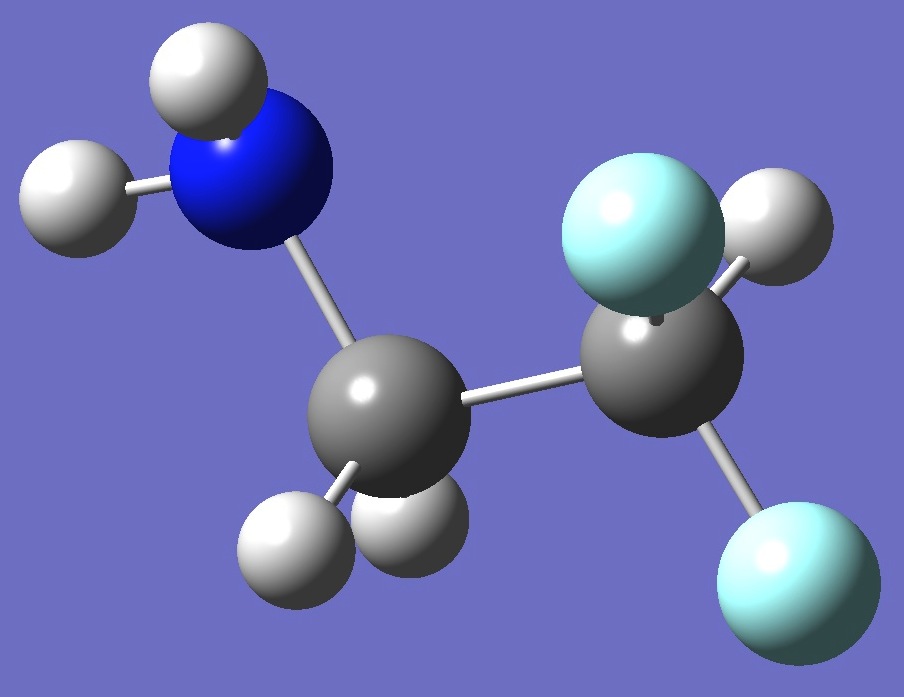
Conformer I
|
|
|
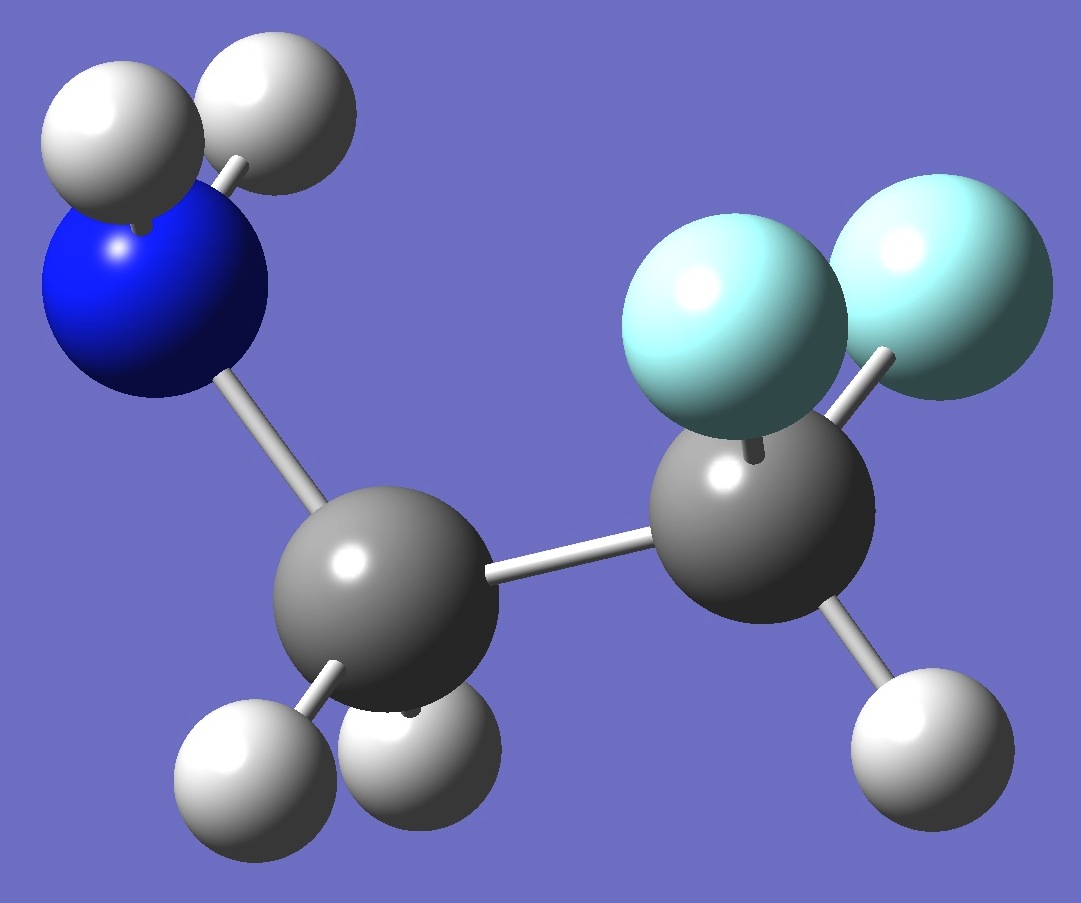
Conformer III
|
|
|
|
EIII < EI
|
|
|
|
by 0.90 kJ/mole
|
|
|
at MP2/aug-cc-pVTZ
|
|
|
level of theory.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculated and experimental nitrogen
nqcc's are compared in Tables 1 and 2. Rotational constants and electric dipole moments are compared in
Table 3. Structure parameters are given here in Z-matrix format.
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz.
RMS is the root mean square difference between calculated and
experiment diagonal nqcc's (percent of average magnitude of
experimental nqcc's). RSD is the calibration residual standard
deviation of the B3PW91/6-311+G(df,pd) model for calculation of the efg's/nqcc's.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N nqcc's in 2,2-Difluoroethylamine, conformer I (MHz). Calculation was made
on (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
2.698
|
|
2.702
|
|
3.1(10)
|
|
|
Xbb
|
|
2.055
|
|
2.054
|
|
2.5(6)
|
|
|
Xcc
|
-
|
4.754
|
-
|
4.756
|
-
|
5.6
|
|
|
Xab
|
|
0.462
|
|
0.465
|
|
|
|
|
Xac |
|
0.395
|
|
0.386
|
|
|
|
|
Xbc |
-
|
0.425
| -
|
0.405
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.599 (16.0 %)
|
|
0.597 (16.0 %)
|
|
|
|
|
RSD
|
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx
|
|
2.943 |
|
2.948
|
|
|
|
|
Xyy
|
|
1.861
|
|
1.855
|
|
|
|
|
Xzz
|
-
|
4.804
|
-
|
4.803
|
|
|
|
|
ETA
|
-
|
0.225
|
-
|
0.228
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N nqcc's in 2,2-Difluoroethylamine, conformer III (MHz). Calculation was made
on (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
4.032
|
-
|
4.017
|
-
|
3.77(17)
|
|
|
Xbb
|
|
1.780
|
|
1.772
|
|
2.78(17)
|
|
|
Xcc
|
|
2.252
|
|
2.245
|
|
0.99
|
|
|
Xac |
|
2.161
|
|
2.182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.942 (37.5 %)
|
|
0.940 (37.4 %)
|
|
|
|
|
RSD
|
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx
|
|
2.923
|
|
2.930
|
|
|
|
|
Xyy
|
|
1.780
|
|
1.772
|
|
|
|
|
Xzz
|
-
|
4.704
|
-
|
4.702
|
|
|
|
|
ETA
|
-
|
0.243
|
-
|
0.246
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3. 2,2-Difluoroethylamine. Rotational Constants (MHz) and Dipole Moments * (D). ropt(1) = MP2/6-311+G(3df,3pd), ropt(2) = MP2/aug-cc-pVTZ. |
|
|
|
|
|
|
Conf I
|
ropt(1) |
ropt(2) |
Expt [1]
|
|
|
|
|
|
|
A
|
9043.1
|
9003.1
|
8987.2718(51)
|
|
B
|
3738.9
|
3727.3
|
3699.8821(19)
|
|
C
|
2855.7
|
2845.8
|
2828.9179(18)
|
|
µa
|
2.13
|
2.15
|
2.042(15)
|
|
µb |
1.04
|
1.06
|
1.101(29)
|
|
µc |
0.46
|
0.45
|
~ 0
|
|
µtot |
2.41
|
2.44
|
2.320(27)
|
|
|
|
|
|
|
Conf III
|
|
|
|
|
|
|
|
|
|
A
|
7141.0
|
7116.0
|
7125.170(12)
|
|
B
|
4236.3
|
4216.0
|
4185.890(13)
|
|
C
|
3474.6
|
3457.7
|
3441.172(13)
|
|
µa |
0.02
|
0.003
|
0.090(28)
|
|
µb |
0 (symm)
|
0 (symm)
|
0 (symm)
|
|
µc |
1.36
|
1.38
|
1.427(28)
|
|
µtot |
1.36
|
1.38
|
1.430(30)
|
|
|
|
|
|
|
* Calculated by B3PW91/6-311+G(df,pd) method on MP2 ropt structures.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] K.-M.Marstokk and H.Mųllendal, Acta Chem.Scand. A 36,517(1982).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3NH2
|
CH3CH2NH2
| CH2FCH2NH2
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CHF2CH2NH2.html |
|
|
|
|
|
|
Last
Modified 2 Jan 2014 |
|
|
|
|
|
|
|
|
|
|

