|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
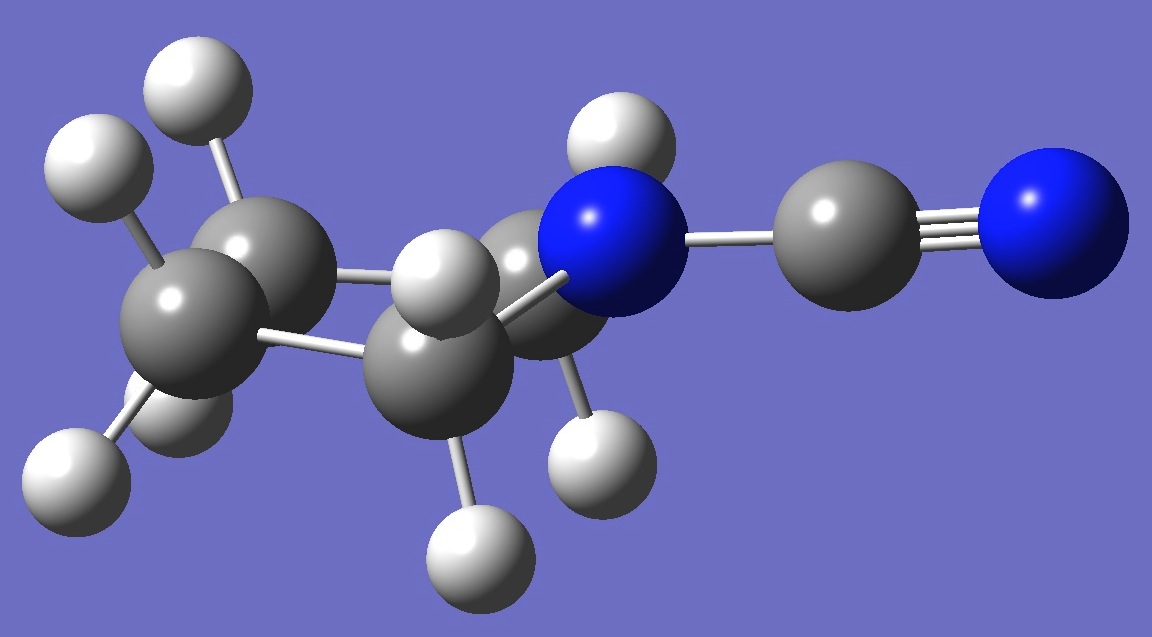
C4H8N-CN
| 
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in N-Cyanopyrrolidine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
microwave spectrum of the equatorial form of N-cyanopyrrolidine was observed by Su and Harmony [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
The 14N nqcc
tensor was calculated here on a molecular
structure given by B3P86/6-31G(3d,3p)
optimization. These calculated nqcc's are given in Table 1,
structure parameters in Table 2, rotational constants and dipole moments in
Table 3.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to
the principal axes of the inertia tensor, subscripts x,y,z to the
principal axes of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscripted parameters.
|
|
|
RSD is the residual standard
deviation
of calibration of the B3PW91/6-311+G(df,pd) model for calculation of
the efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Pyrrolidine Nitrogen nqcc's in N-Cyanopyrrolidine (MHz). Calculation was made on structure given by B3P86/6-31G(3d,3p) optimization.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
|
2.996
|
|
|
|
|
|
Xbb |
|
2.403
|
|
|
|
|
|
Xcc |
-
|
5.399
|
|
|
|
|
|
Xac |
|
0.882
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
3.087
|
|
|
|
|
|
Xyy |
|
2.403 |
|
|
|
|
|
Xzz |
-
|
5.490
|
|
|
|
|
|
ETA |
-
|
0.126
|
|
|
|
|
|
Øz,a |
|
95.93
|
|
|
|
|
|
Øa,NC(6)* |
|
4.61
|
|
|
|
|
|
Øz,NC(6) |
|
100.54
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
* See below for atomic numnering.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrile Nitrogen nqcc's in N-Cyanopyrrolidine (MHz). Calculation was made on structure given by B3P86/6-31G(3d,3p) optimization.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
-
|
3.223
|
|
|
|
|
|
Xbb |
|
2.966
|
|
|
|
|
|
Xcc |
|
0.256
|
|
|
|
|
|
Xac |
|
0.200
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.268
|
|
|
|
|
|
Xyy |
|
2.966
|
|
|
|
|
|
Xzz |
-
|
3.234
|
|
|
|
|
|
ETA |
|
0.834
|
|
|
|
|
|
Øz,a |
|
3.29
|
|
|
|
|
|
Øa,CN |
|
2.63
|
|
|
|
|
|
Øz,CN |
|
0.66
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
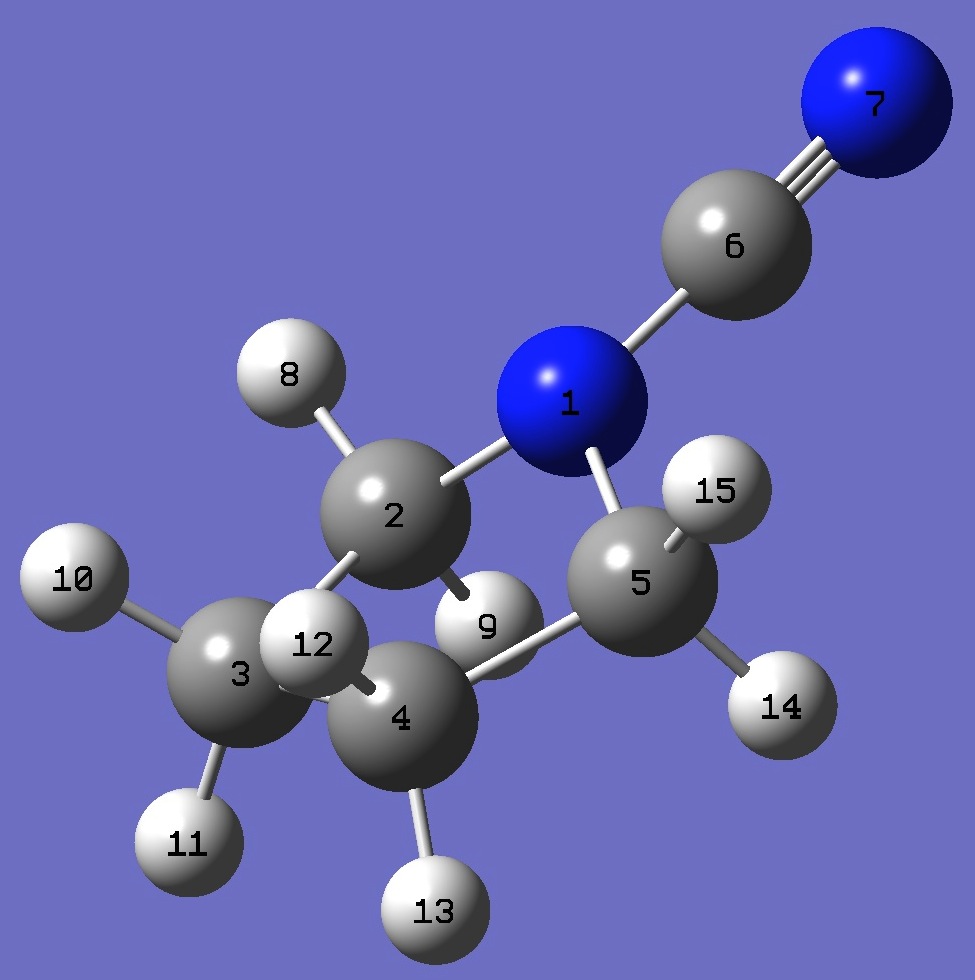
| Table 3. N-Cyanopyrrolidine.
B3P86/6-31G(3d,3p) optimized molecular structure parameters (Å and degrees). |
| |
|
|
|
|
|
|
|
|
|
| 
|
N
C 1 B1
C 2 B2 1 A1
C 3 B3 2 A2 1 D1
C 1 B4 2 A3 3 D2
C 1 B5 5 A4 4 D3
N 6 B6 1 A5 5 D4
H 2 B7 1 A6 6 D5
H 2 B8 1 A7 6 D6
H 3 B9 2 A8 1 D7
H 3 B10 2 A9 1 D8
H 4 B11 3 A10 2 D9
H 4 B12 3 A11 2 D10
H 5 B13 1 A12 6 D11
H 5 B14 1 A13 6 D12
|
|
|
|
|
|
|
| B1 1.46580581
B2 1.53179727
B3 1.54838601
B4 1.46580581
B5 1.32884122
B6 1.16293669
B7 1.09062967
B8 1.09828981
B9 1.09083028
B10 1.09088126
B11 1.09083028
B12 1.09088126
B13 1.09828981
B14 1.09062967
A1 102.74668740
A2 105.73626682
A3 108.50287769
A4 119.99129665
A5 178.01769620
A6 109.88991405
|
A7 110.75472530
A8 110.32992061
A9 110.90513849
A10 110.34783410
A11 112.26200237
A12 110.75472530
A13 109.88991405
D1 -21.83824974
D2 37.79594783
D3 178.90638974
D4 -110.43650949
D5 -57.50843035
D6 62.06570992
D7 97.47961474
D8 -143.77319211
D9 119.30603753
D10 -121.05888286
D11 -62.06570992
D12 57.50843035
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Table 4. N-Cyanopyrrolidine. Rotational Constants (MHz) and Dipole Moments (D).
|
|
|
|
|
|
|
Calc |
Expt. [1] |
|
|
|
|
|
A |
6755
|
67585.05(383)
|
|
B |
1898
|
1919.54(5)
|
|
C |
1559
|
1583.84(5)
|
|
|
|
|
|
|µa|
|
5.50
|
|
|
|µc| |
0.46
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] C.F.Su and M.D.Harmony, J.Mol.Spectrosc. 112,328(1985).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyrrolidine
| N-Methylpyrrolidine
|
N-Cyanomethanimine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CNpyrrolidine.html |
|
|
|
|
|
|
Last
Modified 26 Jan 2014
|
|
|
|
|
|
|
|
|
|
|

