|
|
|
|
|
|
|
|
|
|
|
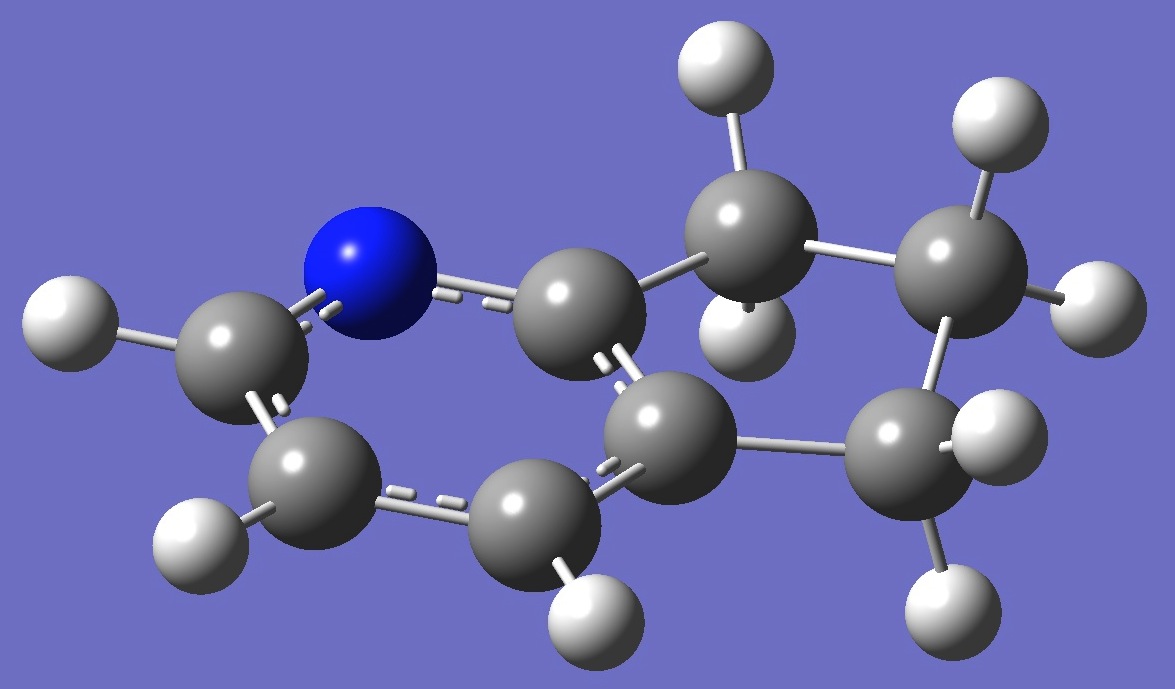
Table
2. 2,3-Cyclopentenopyridine B3P86/6-31G(3d,3p) optimized
structure
parameters (Å and degrees).
|
|
|
|
|
|
|
|
|
|
|
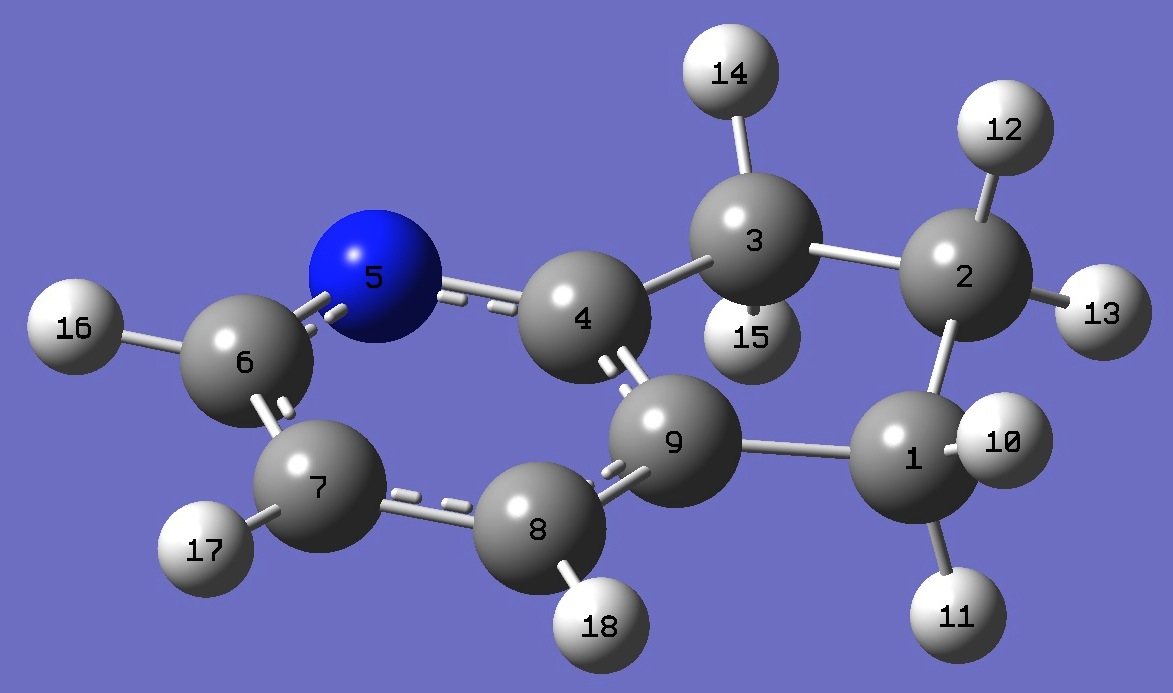
|
C
C,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
N,4,B4,3,A3,2,D2,0
C,5,B5,4,A4,3,D3,0
C,6,B6,5,A5,4,D4,0
C,7,B7,6,A6,5,D5,0
C,8,B8,7,A7,6,D6,0
H,1,B9,9,A8,8,D7,0
H,1,B10,9,A9,8,D8,0
H,2,B11,1,A10,9,D9,0
H,2,B12,1,A11,9,D10,0
H,3,B13,2,A12,1,D11,0
H,3,B14,2,A13,1,D12,0
H,6,B15,5,A14,4,D13,0
H,7,B16,6,A15,5,D14,0
H,8,B17,7,A16,6,D15,0
|
|
|
|
|
|
|
|
|
|
|
|
B1=1.54532992
B2=1.53985444
B3=1.50544586
B4=1.3280745
B5=1.33712998
B6=1.39044374
B7=1.39257501
B8=1.38470916
B9=1.0932392
B10=1.0973679
B11=1.09384774
B12=1.09147308
B13=1.09185205
B14=1.0970143
B15=1.08765487
B16=1.08437334
B17=1.0861259
A1=105.64776471
A2=103.06823264
A3=124.44752851
A4=115.9807775
A5=123.98117283
A6=119.15857962
A7=117.65259617
|
|
A8=113.25777163
A9=110.08211531
A10=109.10264477
A11=112.56150959
A12=113.62311124
A13=111.57999009
A14=115.93978666
A15=119.87706617
A16=120.79040325
D1=25.09131729
D2=165.05507882
D3=179.32795336
D4=-0.206807
D5=-0.13189998
D6=0.35374146
D7=-42.93771711
D8=76.35624752
D9=92.14808742
D10=-148.96053597
D11=146.86395621
D12=-92.37238947
D13=179.60411283
D14=179.69883572
D15=-179.97692179
|
|
|
|
|
|
|
|
|
|
|
|
|