|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cotinine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Cotinine |
|
|
|
[1-Methyl-5-(3-pyridyl)(2-piperidyl)pyrrolidin-2-one]
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Rotational spectra of two conformers
of cotinine have been
investigated by Uriarte et al. [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the N nqcc tensors was
made here on molecular structures optimized at the
B3P86/6-31G(3d,3p) level of theory. These structures are
shown below. Calculated nqcc's are given in Tables 1 and 2. Rotational
constants and dipole moments are given in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Conformer I
|
Conformer II |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
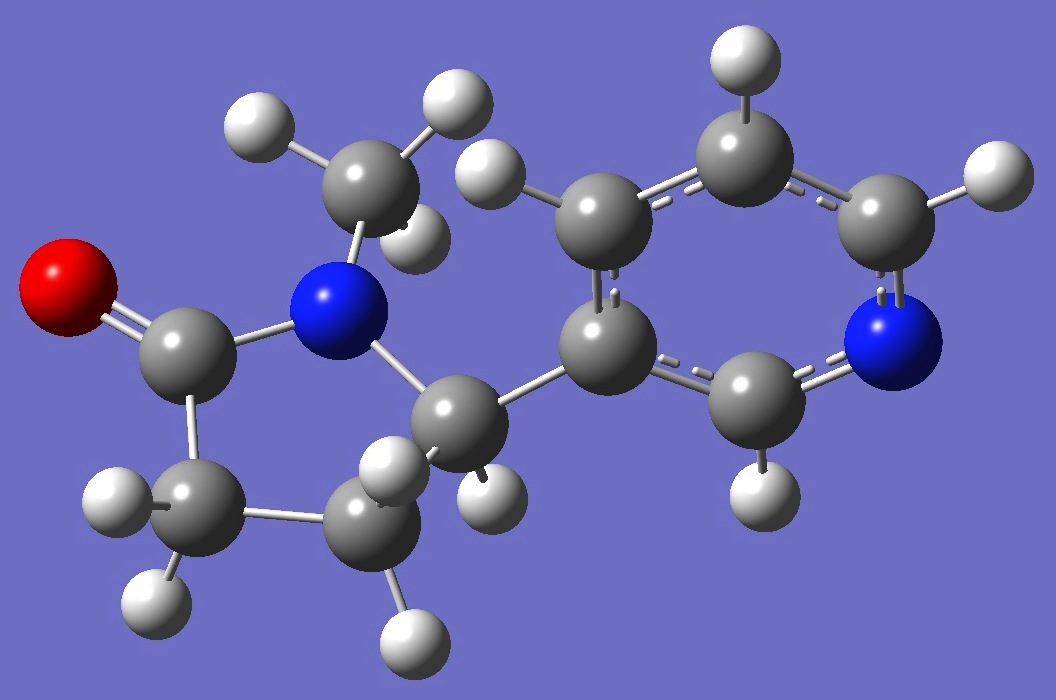 |
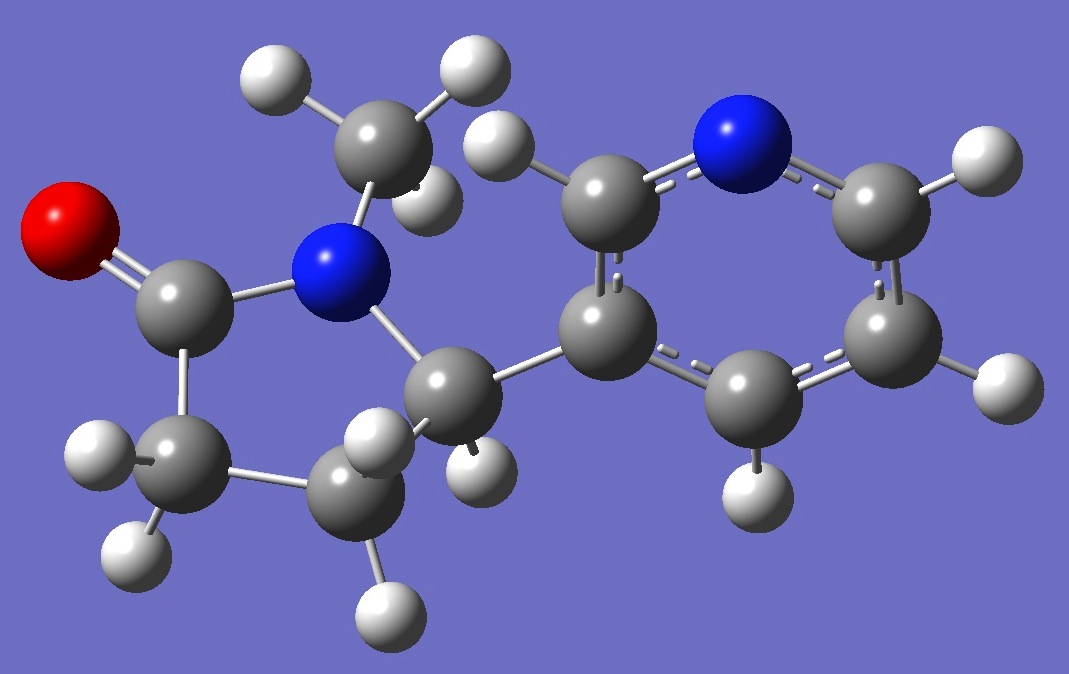 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Optimized
structures of these conformers are given here in Z-matrix format.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c
refer to
the principal axes of the inertia tensor, subscripts x,y,z to the
principal axes of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental diagonal nqcc's (percent
of the average of the magnitudes of experimental nqcc's). RSD is
the residual standard deviation of the B3PW91/6-311+G(df,pd) model for calculation of the
efg's/nqcc's.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. 14N nqcc's in Cotinine I (MHz). N(5) is pyrrolidinic nitrogen, N(19) is pyridinic.
Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc |
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
14N(5) |
Xaa |
|
0.963
|
|
1.09(11)
|
|
|
|
Xbb |
|
1.984
|
|
2.09(25)
|
|
|
|
Xcc |
-
|
2.947
|
-
|
3.17(25)
|
|
|
|
Xab |
-
|
0.752
|
|
|
|
|
|
Xac |
|
2.064
|
|
|
|
|
|
Xbc |
|
1.259
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.786
|
|
|
|
|
|
Xyy |
|
2.392
|
|
|
|
|
|
Xzz |
-
|
4.178
|
|
|
|
|
|
ETA |
|
0.145
|
|
|
|
|
|
|
|
|
|
|
|
|
|
---------------------------------------------------------------------------- |
|
|
|
|
|
|
|
|
|
|
14N(19) |
Xaa |
- |
1.824 |
-
|
1.76(11)
|
|
|
|
Xbb |
|
2.706 |
|
2.31(25)
|
|
|
|
Xcc |
-
|
0.881 |
-
|
0.55(25)
|
|
|
|
Xab |
|
1.004 |
|
|
|
|
|
Xac |
-
|
3.034 |
|
|
|
|
|
Xbc |
|
1.826 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.437 |
|
|
|
|
|
Xyy |
|
3.490 |
|
|
|
|
|
Xzz |
- |
4.927 |
|
|
|
|
|
ETA |
|
0.416 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. 14N nqcc's in Cotinine II (MHz). N(5) is pyrrolidinic nitrogen, N(19) is pyridinic.
Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc |
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
14N(5) |
Xaa |
|
0.890
|
|
0.747(73)
|
|
|
|
Xbb |
|
1.894
|
|
1.45(28)
|
|
|
|
Xcc |
-
|
2.784
|
-
|
2.19(28)
|
|
|
|
Xab |
|
0.861
|
|
|
|
|
|
Xac |
|
2.110
|
|
|
|
|
|
Xbc |
-
|
1.425
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.788
|
|
|
|
|
|
Xyy |
|
2.406
|
|
|
|
|
|
Xzz |
-
|
4.194
|
|
|
|
|
|
ETA |
|
0.147
|
|
|
|
|
|
|
|
|
|
|
|
|
|
---------------------------------------------------------------------------- |
|
|
|
|
|
|
|
|
|
|
14N(19) |
Xaa |
|
1.217
|
|
1.215(65)
|
|
|
|
Xbb |
|
1.344
|
|
|
|
|
|
Xcc |
-
|
2.561
|
|
|
|
|
|
Xab |
|
0.731
|
|
|
|
|
|
Xac |
|
0.994
|
|
|
|
|
|
Xbc |
-
|
2.561
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.435
|
|
|
|
|
|
Xyy |
|
3.525
|
|
|
|
|
|
Xzz |
-
|
4.960
|
|
|
|
|
|
ETA |
|
0.421
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3. Cotinine.
Rotational constants (MHz) and dipole moments (D). Calc is
on the
B3P86/6-31G(3d,3p) opt structure. |
|
|
|
|
|
______________Conformer I_______________ |
|
|
|
|
|
|
Calc |
Expt [1]
|
|
|
|
|
|
A |
1909.8
|
1911.19(12)
|
|
B |
481.1
|
478.56272(41)
|
|
C |
452.2
|
449.43077(45)
|
|
|
|
|
|
|µa| |
1.64
|
|
|
|µb| |
1.16
|
|
|
|µc| |
0.51
|
|
|
|
|
|
|
______________Conformer II_______________ |
|
|
|
|
|
A |
1891.5
|
1893.66(11)
|
|
B |
485.6
|
482.65540(38)
|
|
C |
454.9
|
452.49336(39)
|
|
|
|
|
|
|µa| |
2.78
|
|
|
|µb| |
2.93
|
|
|
|µc| |
2.83
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyridine
|
Anabasine |
Nicotine |
N-Methylpyrrolidine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] I.Uriarte, C.Perez,
E.Caballero-Mancebo, F.J.Basterretxea, A.Lesarri, J.A.Fernández, and E.J.Cocinero, Chem.Eur.J. 23(30),7238(2017).
|
|
|
|
|
|
|
|
|
|
|
|
|
I.Uriarte, P.Ecija, E.J.Cocinero, C.Perez,
E.Caballero-Mancebo, and A.Lesarri, Abstract TD05, 70th International
Symposium on Molecular Spectroscopy, Champaign-Urbana, 2015.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cotinine.html |
|
|
|
|
|
|
Last
Modified 20 July 2015 |
|
|
|
|
|
|
|
|
|
|

