|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCCCHCHCN
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in t-Cyanovinylacetylene |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the nitrogen
nqcc's in t-cyanovinylacetylene was made on a molecular structure derived
ab initio
as discussed below. These are compared with the experimental
nqcc's of Thornwirth et al. [1] in Tables 1 and 2. Structure
parameters are given in Table 3, rotational constants in Table 4.
A link is provided below to the Gaussian input file in Z-Matrix
format. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the principal axes of the inertia
tensor, subscripts x,y,z to the principal axes of the nqcc tensor.
The nqcc y-axis is chosen coincident with the inertia c-axis, these
are perpendicular to the plane of the molecule. Ø (degrees)
is the angle between its subscripted parameters. ETA = (Xxx
- Xyy)/Xzz. |
|
|
|
|
|
|
|
|
|
|
|
|
RMS is the root mean square
difference between calculated and experimental nqcc's (percentage of
average experimental nqcc). RSD is the residual stand deviation
of calibration of the B3PW91/6-311+G(df,pd) model for calculation of
the nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc's in t-Cyanovinylacetylene (MHz). Calculation was
made on the structure given below in Table 3. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
3.924 |
- |
3.90(2) |
|
|
|
Xbb |
|
1.851 |
|
1.82(3) |
|
|
|
Xcc |
|
2.074 |
|
2.08 |
|
|
|
|Xab| |
|
1.536 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.023 (0.89 %) |
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.234 |
|
2.21 * |
|
|
|
Xyy |
|
2.074 |
|
2.08 |
|
|
|
Xzz |
- |
4.308 |
- |
4.29 |
|
|
|
ETA |
- |
0.037 |
- |
0.030 |
|
|
|
Øz,a |
|
14.00 |
|
14.12 |
|
|
|
Øa,CN |
|
13.48 |
|
13.48 |
|
|
|
Øz,CN |
|
0.52 |
|
0.64 |
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
* Calculated here from the diagonal experimental nqcc's and
the calculated off-diagonal nqcc. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen
nqcc's in t-Cyanovinylacetylene (MHz). Calculation was
made on the structure given below in Table 3, but with linear HCCC and CCN. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
3.919 |
- |
3.90(2) |
|
|
|
Xbb |
|
1.846 |
|
1.82(3) |
|
|
|
Xcc |
|
2.073 |
|
2.08 |
|
|
|
|Xab| |
|
1.543 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.019 (0.74 %) |
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.234 |
|
2.21 * |
|
|
|
Xyy |
|
2.073 |
|
2.08 |
|
|
|
Xzz |
- |
4.306 |
- |
4.29 |
|
|
|
ETA |
- |
0.037 |
- |
0.030 |
|
|
|
Øz,a |
|
14.08 |
|
14.17 |
|
|
|
Øa,CN |
|
13.90 |
|
13.90 |
|
|
|
Øz,CN |
|
0.18 |
|
0.27 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from the diagonal experimental nqcc's and
the calculated off-diagonal nqcc. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
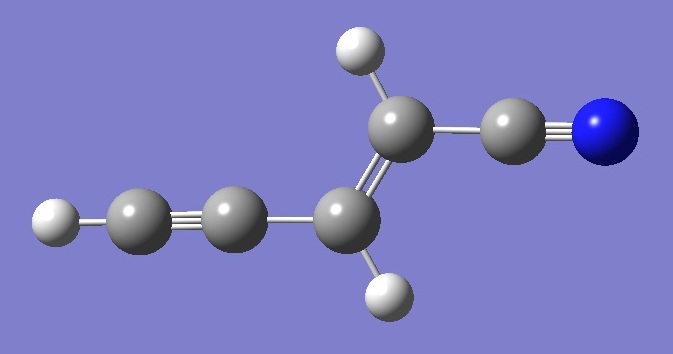
Molecular Structure
|
|
|
|
|
|
|
|
|
|
|
|
|
The molecular structure was optimized
at the MP2/6-311G(d,p) level of theory assuming Cs symmetry.
The optimized CC single and CC double bond lengths were then
corrected using the equation obtained from linear regression analysis
of the data given in Table IX of Ref.[3]. The CH bond lengths were
corrected using r = 1.001 ropt, where ropt is obtained
by MP2/6-31G(d,p) optimization [4]. The CC triple bond was determined
by B3LYP/6-31+G(df,3pd) optimization and corrected as described in summary/acetylenes. Interatomic angles used
in the calculation are those given by MP2/6-311G(d,p) optimization. Structure
parameters thus obtained are shown in Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
Atomic Numbering |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| HC(5)C(4) |
|
|
|
H |
|
|
|
C(3) |
= |
C(2) |
|
|
|
H |
|
|
|
C(1)N |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| Table 3. Molecular structure
parameters (Å and degrees). |
| |
|
|
|
C(1)N |
1.158 |
|
C(2)C(1) |
1.427 |
|
C(3)C(2) |
1.347 |
|
C(2)H |
1.083 |
|
C(3)H |
1.085 |
|
C(3)C(4) |
1.421 |
|
C(4)C(5) |
1.206 |
|
C(5)H |
1.062 |
|
C(2)C(1)N |
179.23 |
|
C(3)C(2)C(1) |
121.71 |
|
C(3)C(2)H |
120.82 |
|
C(2)C(3)H |
119.55 |
|
C(2)C(3)C(4) |
122.57 |
|
C(3)C(4)C(5) |
178.59 |
|
C(4)C(5)H |
179.58 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Gaussian Input: Z-Matrix |
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
| Table 4. Rotational Constants
(MHz). |
| |
|
|
|
|
|
Calc. ropt |
Expt. [1,2] |
|
|
|
|
|
A |
46352 |
46199(18) |
|
B |
1473.5 |
1472.1538(2) |
|
C |
1428.1 |
1426.2484(2) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] S.Thorwirth, M.C.McCarthy, J.B.Dudek,
and P.Thaddeus, J.Mol. Spectrosc. 225,93(2004). |
|
|
[2] J.August, H.W.Kroto, D.McNaughton,
K.Phillips, and D.R.M.Walton, J.Mol.Spectrosc. 130,424(1988). |
|
|
[3] J.Demaison, J.Cosléou, R.Bocquet,
and A.G.Lesarri, J.Mol.Spectrosc. 167,400(1994). |
|
|
[4] J.Demaison and G.Wlodarczak, Structural
Chem. 5,57(1994). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCCCHCHCN.html |
|
|
|
|
|
|
Last
Modified 25 June 2004 |
|
|
|
|
|
|
|
|
|
|