|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
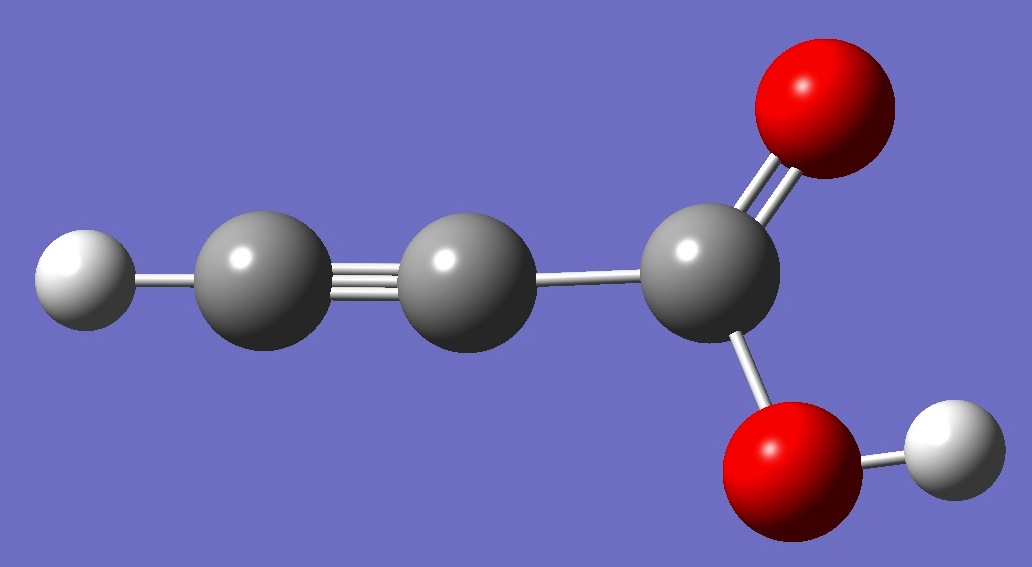
H-CC-C(=O)OH
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deuterium |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Propiolic Acid, d1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the deuterium nqcc tensors in monodeuterated propiolic acid was made here on ropt molecular structures given by
MP2/6-311+G(3df,3pd) and MP2/aug-cc-pVTZ optimization. These are compared with the
experimental nqcc's [1] in Tables 1 and 2. Structure parameters are given in Table 3, rotational constants in Table 4.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor.
Ø (degrees) is the angle between its subscripted
parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental nqcc's (percentage of the
average of the magnitudes of the experimental nqcc's). RSD is the
calibration residual standard deviation of the B3LYP/6-31G(df,3p) model for calculation of deuterium efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. D nqcc's in H-CC-C(=O)OD (kHz). Calculation was made
on the (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt molecular structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
260.8 |
|
255.3 |
|
256.5(33) *
|
|
|
Xbb |
-
|
110.7 |
-
|
108.3 |
-
|
112.6(45) *
|
|
|
Xcc |
-
|
150.1 |
-
|
147.0 |
-
|
143.9(45) *
|
|
|
|Xab| |
|
61.5
|
|
59.7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
4.4 (2.6 %)
|
3.1 (1.8 %)
|
|
|
|
RSD |
|
1.1 (0.86 %)
|
1.1 (0.86 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
-
|
120.6 |
-
|
117.8 |
|
|
|
|
Xyy |
-
|
150.1 |
-
|
147.0 |
|
|
|
|
Xzz |
|
270.7 |
|
264.9 |
|
|
|
|
ETA |
|
0.109 |
|
0.110 |
|
|
|
|
Øz,a
|
|
9.17
|
|
9.10
|
|
|
|
|
Øa,OD |
|
10.62
|
|
10.56
|
|
|
|
|
Øz,OD |
|
1.45
|
|
1.46
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental 1.5Xaa = 0.3848(49) and 0.25(Xbb - Xcc) = 0.00785(211) MHz [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. D nqcc's in D-CC-C(=O)OH (kHz). Calculation was made
on the (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt molecular structures. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1)
|
|
Calc (2)
|
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
212.9 |
|
212.6 |
|
205.3(27) *
|
|
|
Xbb |
-
|
108.7 |
-
|
108.5 |
|
|
|
|
Xcc |
-
|
104.2 |
-
|
104.1 |
|
|
|
|
|Xab| |
|
5.4
|
|
5.7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
7.6 (3.7 %)
|
7.3 (3.6 %)
|
|
|
|
RSD |
|
1.1 (0.86 %)
|
1.1 (0.86 %) |
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
-
|
108.8 |
-
|
108.6 |
|
|
|
|
Xyy |
-
|
104.2 |
-
|
104.1 |
|
|
|
|
Xzz |
|
213.0 |
|
212.7 |
|
|
|
|
ETA |
|
0.0213 |
|
0.0214 |
|
|
|
|
Øz,a
|
|
0.96
|
|
1.014
|
|
|
|
|
Øa,CD |
|
0.96
|
|
1.017
|
|
|
|
|
Øz,CD |
|
0.00
|
|
0.003
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental 1.5Xaa = 0.3080(40) MHz [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
| Table 3. Propiolic Acid molecular structure parameters, ropt(1) = MP2/6-311+G(3df,3pd) optimization and ropt(2) = MP2/aug-cc-pVTZ optimization (Å
and degrees). |
| |
|
|
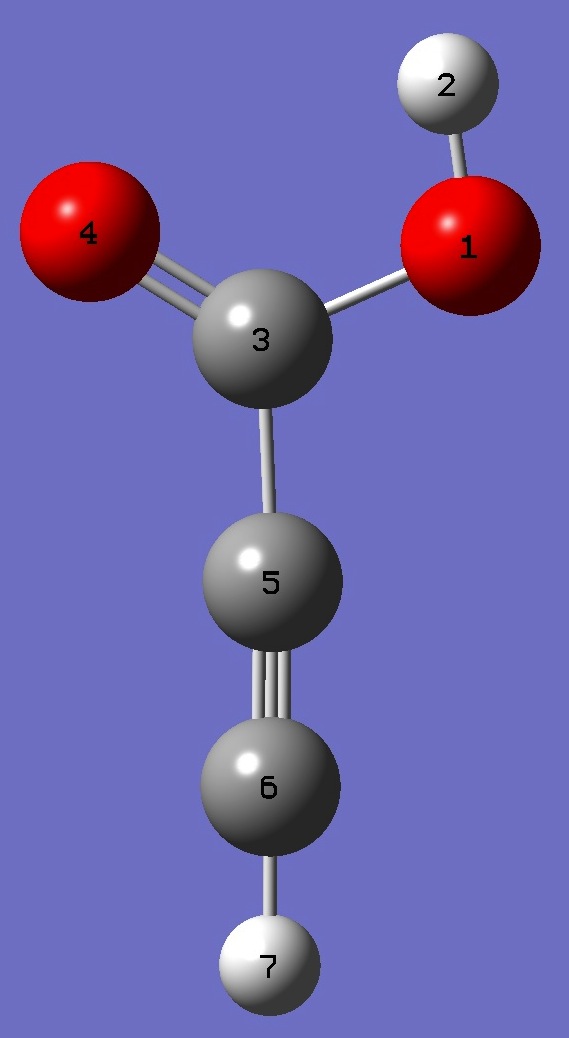
|
O
H,1,B1
C,1,B2,2,A1
O,3,B3,1,A2,2,D1,0
C,3,B4,1,A3,4,D2,0
C,5,B5,2,A4,4,D3,0
H,6,B6,4,A5,2,D4,0
|
|
|
| ropt(1) |
ropt(2) |
|
|
B1=0.96815607
B2=1.34618353
B3=1.20674381
B4=1.44665384
B5=1.21305172
B6=1.06234116
A1=105.75550831
A2=124.15443066
A3=111.39565131
A4=161.14605072
A5=161.75136421
D1=0.
D2=180.
D3=180.
D4=180.
|
B1=0.97119556
B2=1.34964818
B3=1.21003443
B4=1.44601681
B5=1.21427027
B6=1.0625963
A1=105.73105785
A2=124.12112095
A3=111.3002228
A4=161.1340831
A5=161.82450926
D1=0.
D2=180.
D3=180.
D4=180.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4. Propiolic Acid rotational constants (MHz). ropt(1) = MP2/6-311+G(3df,3pd), ropt(2) = MP2/aug-cc-pVTZ optimized structures. |
| |
|
|
|
|
|
|
|
|
ropt(1) |
ropt(2) |
Expt [1] |
|
|
|
|
|
|
| HCCC(=O)OD
|
A
|
11848.4
|
11788.7
|
11858.44934(132)
|
|
|
B
|
4003.1
|
3998.0
|
4015.71252(41)
|
|
|
C
|
2992.2
|
2985.5
|
2995.59587(48)
|
|
|
|
|
|
|
DCCC(=O)OH
|
A
|
12100.2
|
12040.2
|
12110.01758(217)
|
|
|
B
|
3805.7
|
3801.0
|
3819.67859(110)
|
|
|
C
|
2895.1
|
2889.0
|
2899.60416(55)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] M.Sun, B.Sargus,
S.J.Carey, and S.G.Kukolich, Abstract RK11, 68th International
Symposium on Molecular Spectroscopy, June 17-21, 2013.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HC(=O)OH
| HCCH
|
CF3C(=O)OH
|
Salicylaldehyde
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Deuterium |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCCCOOH.html |
|
|
|
|
|
|
Last
Modified 7 July 2013 |
|
|
|
|
|
|
|
|
|
|

