| |
|||||||
| Table 1. Nitrogen nqcc's in axial N-methy-piperidine (MHz). Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14N | Xaa | - |
0.733 |
||||
| Xbb | 2.736 |
||||||
| Xcc | - |
2.003 |
|||||
| |Xac| | 4.322 |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | 2.999 |
||||||
| Xyy | 2.736 | ||||||
| Xzz | - |
5.736 |
|||||
| ETA |
- |
0.0459 |
|||||
| Øz,a |
49.18 |
||||||
| Øa,NMe | 55.87 |
||||||
| Øz,NMe | 105.05 |
||||||
| |
|||||||
| |
|||||||
| Table 2. Nitrogen nqcc's in equatorial N-methy-piperidine (MHz). Calculation was made
on B3P86/6-31G(3d,3p) ropt structure. |
|||||||
| |
|||||||
| Calc |
Expt |
||||||
| |
|||||||
| 14N | Xaa | 2.669 |
|||||
| Xbb | 2.579 |
||||||
| Xcc | - |
5.248 |
|||||
| |Xac| | 0.725 |
||||||
| RSD |
0.030 (1.3 %) |
||||||
| Xxx | 2.735 |
||||||
| Xyy | 2.579 |
||||||
| Xzz | - |
5.314 |
|||||
| ETA |
- |
0.0293 |
|||||
| Øz,a |
95.19 |
||||||
| Øa,NMe | 12.23 | ||||||
| Øz,NMe | 107.42 |
||||||
| |
|||||||
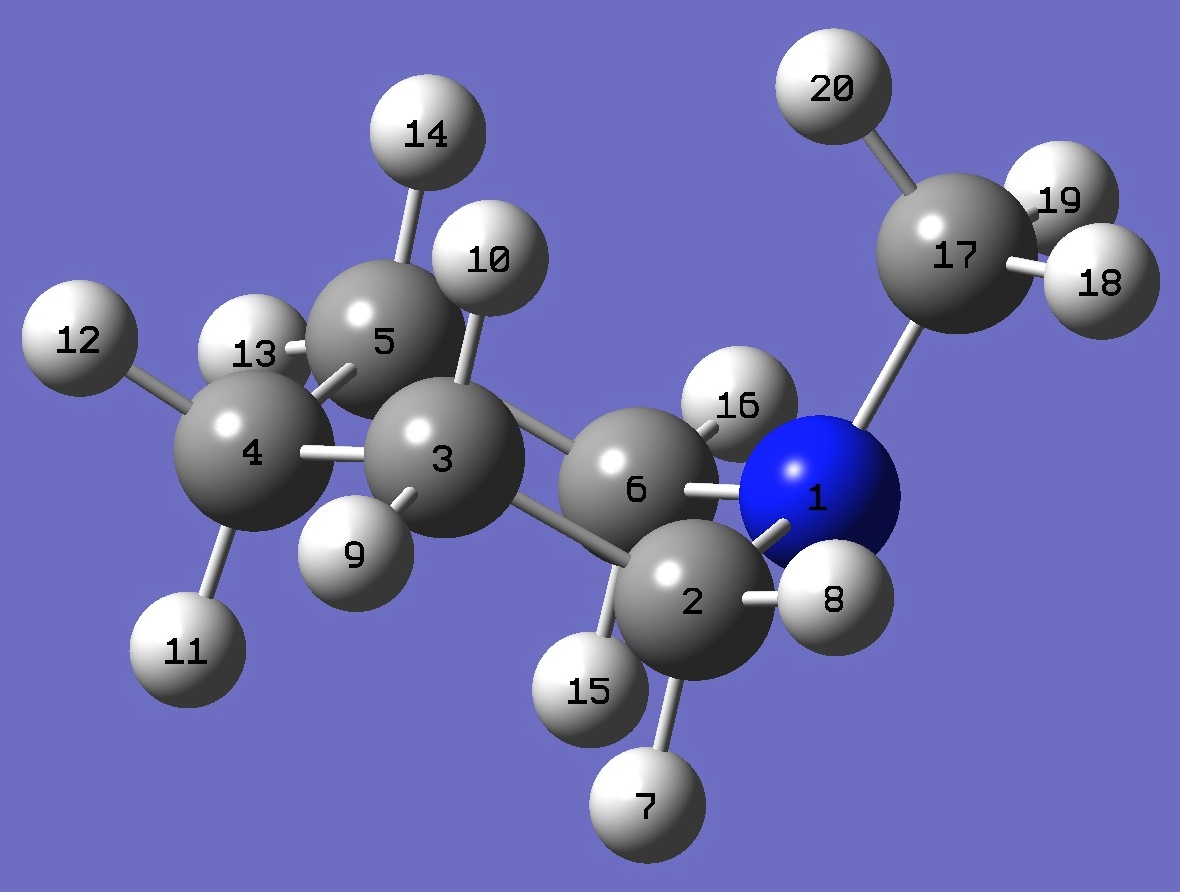
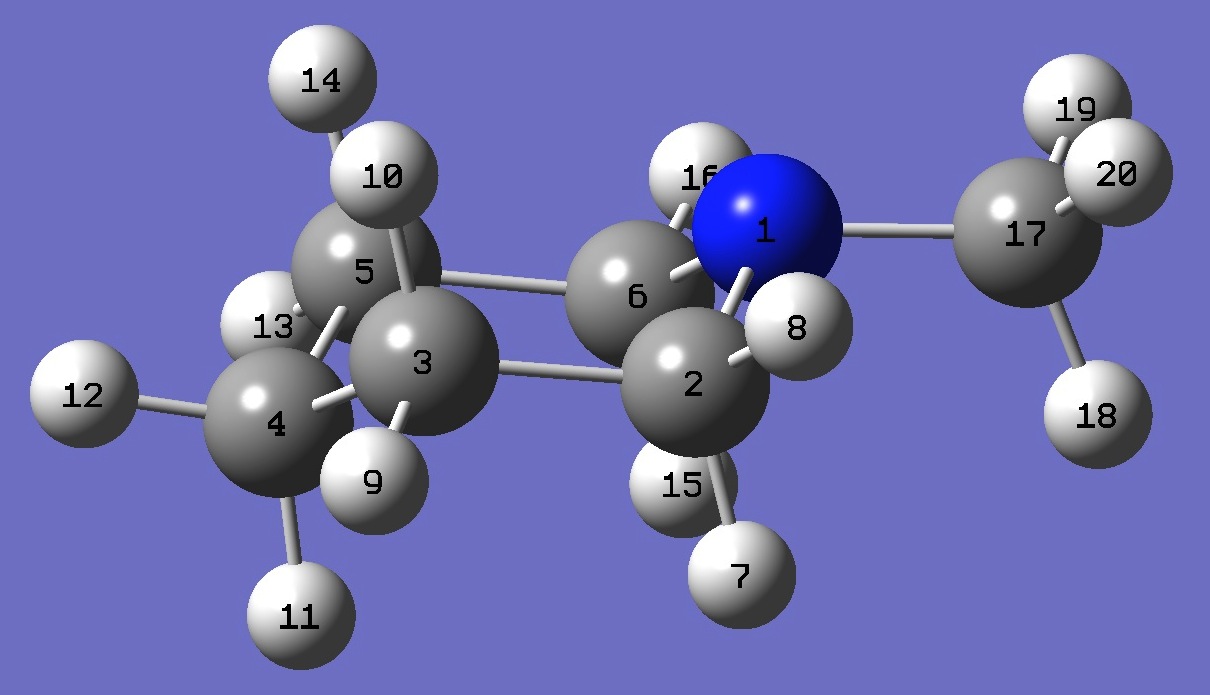
| Table 3. N-Methyl-Piperidine. B3P86/6-31G(3d,3p) optimized structure parameters (Å and degrees). Atomic numbering is shown above. | |||||
| |
|
||||
| |
|||||
| # B3PW91/6-311+G(df,pd) prop scf=tight N-Methyl-Piperidine 0 1 N C,1,B1 C,2,B2,1,A1 C,3,B3,2,A2,1,D1,0 C,4,B4,3,A3,2,D2,0 C,1,B5,2,A4,3,D3,0 H,2,B6,1,A5,6,D4,0 H,2,B7,1,A6,6,D5,0 H,3,B8,2,A7,1,D6,0 H,3,B9,2,A8,1,D7,0 H,4,B10,3,A9,2,D8,0 H,4,B11,3,A10,2,D9,0 H,5,B12,4,A11,3,D10,0 H,5,B13,4,A12,3,D11,0 H,6,B14,1,A13,2,D12,0 H,6,B15,1,A14,2,D13,0 C,1,B16,6,A15,5,D14,0 H,17,B17,1,A16,6,D15,0 H,17,B18,1,A17,6,D16,0 H,17,B19,1,A18,6,D17,0 |
|||||
| axial |
equatorial |
||||
| B1 1.45661677 B2 1.53135457 B3 1.52816428 B4 1.52816428 B5 1.45661677 B6 1.09868575 B7 1.09470278 B8 1.09527674 B9 1.09687172 B10 1.098635 B11 1.09462142 B12 1.09527674 B13 1.09687172 B14 1.09868575 B15 1.09470278 B16 1.44793297 B17 1.09398488 B18 1.09398488 B19 1.10378044 A1 114.16994872 A2 110.25173368 A3 110.63937445 A4 110.98971346 A5 107.49423365 A6 108.4050048 A7 109.73440392 A8 109.86892245 A9 109.01502569 A10 110.80581399 A11 110.73980101 A12 109.73065471 A13 107.49423365 A14 108.4050048 A15 113.82064771 A16 109.25205949 A17 109.25205949 A18 115.43684886 D1 54.49628382 D2 -52.97889192 D3 -54.42856471 D4 66.31817414 D5 -178.57107996 D6 176.72941794 D7 -66.56493978 D8 66.89293854 D9 -176.28861102 D10 174.61736503 D11 -68.16484301 D12 -66.31817414 D13 178.57107996 D14 -75.53036772 D15 -174.49701742 D16 -56.97304125 D17 64.26497067 |
B1 1.45445960 B2 1.52222276 B3 1.52531362 B4 1.52531362 B5 1.45445960 B6 1.11033922 B7 1.09478062 B8 1.09465824 B9 1.09581256 B10 1.09792117 B11 1.09435549 B12 1.09465824 B13 1.09581256 B14 1.11033922 B15 1.09478062 B16 1.44529301 B17 1.10689788 B18 1.09365760 B19 1.09365760 A1 111.14603012 A2 110.73787163 A3 110.16712256 A4 111.31063788 A5 110.99425550 A6 108.16444133 A7 109.54955222 A8 108.72981190 A9 109.26357400 A10 110.72902502 A11 110.79819194 A12 109.70549899 A13 110.99425550 A14 108.16444133 A15 111.41657683 A16 113.13803449 A17 109.89897918 A18 109.89897918 D1 56.35054280 D2 -52.87238159 D3 -59.74649609 D4 62.23816489 D5 179.02413595 D6 178.85392927 D7 -64.25222523 D8 67.17154091 D9 -175.70320042 D10 174.64550072 D11 -67.14863059 D12 -62.23816489 D13 -179.02413595 D14 -175.22213265 D15 -62.48609677 D16 58.15548596 D17 176.87232049 |
||||
| Table
4. N-Methyl-Piperidine. Rotational Constants (MHz) and
Electric Dipole Moments (D) calculated on B3P86/6-31G(3d,3p) optimized
structures. |
|||||
| axial |
Expt. |
equatorial |
Expt. |
||
| A |
3749.1 |
4472.4 |
|||
| B |
2625.1 |
2322.9 |
|||
| C |
1979.5 |
1676.7 |
|||
| |µa| |
0.61 |
0.09 |
|||
| |µb| | 0 (symmetry) | 0 (symmetry) |
|||
| |µc| | 0.64 |
0.48 |
|||