|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
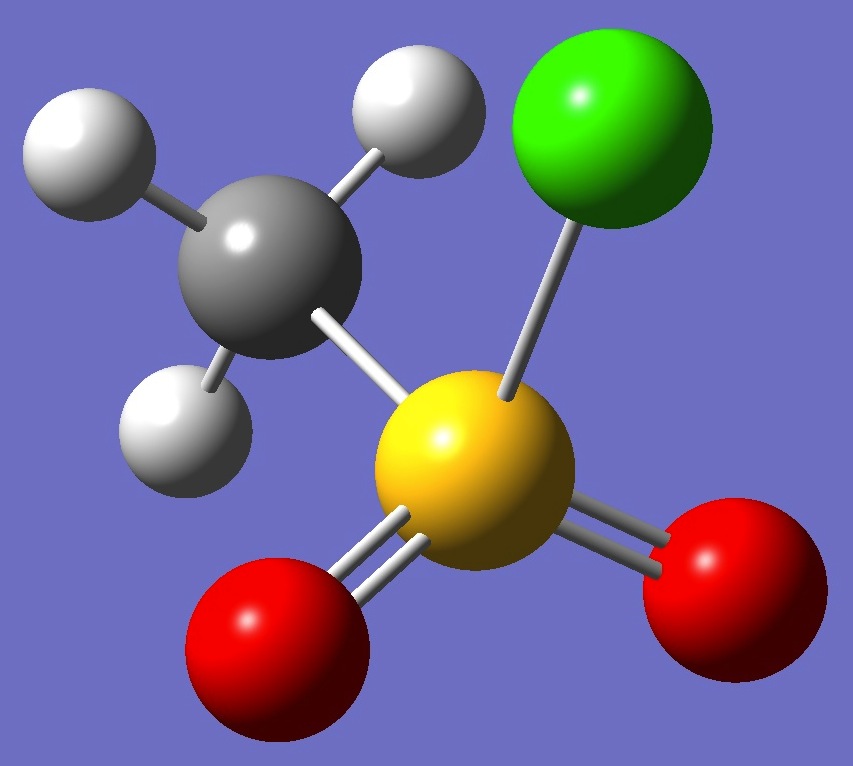
CH3SO2Cl
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Methane Sulfonyl Chloride |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The microwave spectrum of
methane sulfonyl chloride was observed by van Eijck et al. [1].
An electron diffraction molecular structure was derived by Hargittai
and Hargittai [2].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine nqcc
tensors was made here on the electron diffraction structure (red), and on a structure given by MP2/6-311+G(3df,3pd) optimization (ropt).
These nqcc's are compared with the experimental values [1] in Tables 1
and 2. Structure parameters are given in Table 3,
rotational constants in Table 4.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to
the principal axes of the inertia tensor, subscripts x,y,z to the
principal axes of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscript parameters.
|
|
|
RMS is the root mean square difference between calculated and experimental nqcc's. RSD is the residual standard
deviation
of calibration of the B1LYP/TZV(3df,2p) model for calculation of
the efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 35Cl nqcc's in Methane Sulfonyl Chloride
(MHz). Calculation was made
on red and ropt molecular structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /red
|
|
Calc /ropt
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
66.80
|
-
|
66.48
|
-
|
65.7(4)
|
|
|
Xbb |
|
30.57
|
|
30.56
|
|
29.9(3)
|
|
|
Xcc |
|
36.23
|
|
35.92
|
|
35.8(3)
|
|
|
Xab |
|
5.69
|
|
6.44
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.78 (1.8 %)
|
|
0.59 (1.3 %)
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
30.91
|
|
30.98
|
|
|
|
|
Xyy |
|
36.23
|
|
35.92
|
|
|
|
|
Xzz |
-
|
67.13
|
-
|
66.90
|
|
|
|
|
ETA |
|
0.0792
|
|
0.0738
|
|
|
|
|
Øz,a |
|
3.33
|
|
3.78
|
|
|
|
|
Øa,SCl |
|
4.43
|
|
3.99
|
|
|
|
|
Øz,SCl |
|
1.10
|
|
0.21
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 37Cl nqcc's in Methane Sulfonyl Chloride
(MHz). Calculation was made
on red and ropt molecular structures.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc /red
|
|
Calc /ropt
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
52.66
|
-
|
52.40
|
-
|
51.6(5)
|
|
|
Xbb |
|
24.11
|
|
24.09
|
|
23.8(4)
|
|
|
Xcc |
|
28.55
|
|
28.31
|
|
27.8(4)
|
|
|
Xab |
|
4.35
|
|
4.99
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS
|
|
0.77 (2.2 %)
|
|
0.57 (1.7 %)
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
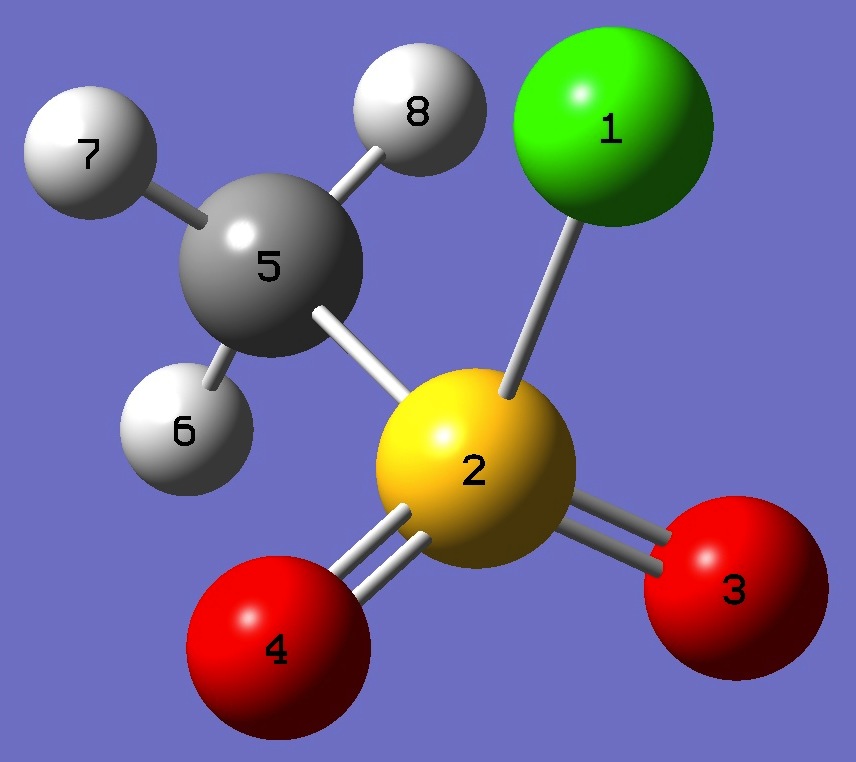
Table 3. Methane Sulfonyl Chloride
(MHz). Molecular structure parameters, red and ropt (Å and degrees).
|
|
|
|
|
|
|
|
|
red
|
ropt
|
|
|
|
|
|
|
SCl
|
2.046(4)
|
2.0497
|
S=O
|
1.424(3)
|
1.4277
|
SC
|
1.763(5)
|
1.7581
|
CH(6)
|
1.101(13)
|
1.0879
|
| CH(7,8) |
1.101(13) |
1.0845
|
CSCl
|
101.0(15)
|
99.56
|
ClS=O
|
107.1(7)
|
106.91
|
O=S=O
|
120.8(24)
|
122.07
|
| SCH(6) |
|
105.40
|
| SCH(7.8) |
|
108.41
|
|
|
H(6)H(7,8)
|
112.0(20)
|
111.11
|
|
|
H(7)H(8)
|
112.0(20)
|
112.08
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
Table 4. Methane Sulfonyl Chloride, 35Cl species.
Rotational Constants (MHz).
|
|
|
|
|
|
|
|
Calc /red
|
Calc /ropt
|
Expt [1]
|
|
|
|
|
|
|
A |
4661
|
4634
|
4636.421(5)
|
|
B |
2776
|
2779
|
2787.938(5)
|
|
C |
2674
|
2690
|
2682.889(5)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] B.P. van Eijck, A.J.Korthof, and F.C.Mijlhoff, J.Mol.Struct. 24,222(1975).
|
|
|
[2] M.Hargittai and I.Hargittai, J.Chem.Phys. 59,2513(1973).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
SO2ClCH3.html |
|
|
|
|
|
|
Last
Modified 24 May 2014 |
|
|
|
|
|
|
|
|
|
|

