|
| |
|
|
|
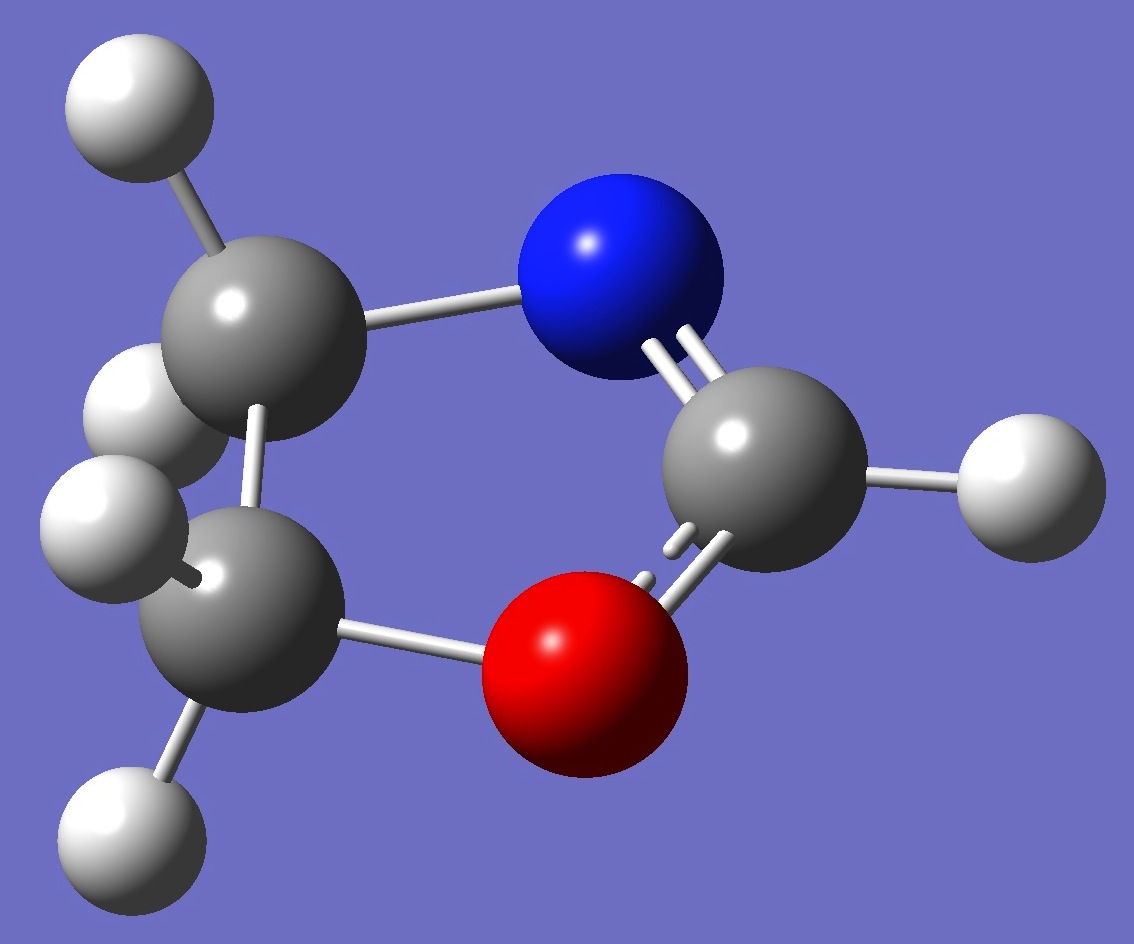
Table 2. 2-Oxazoline.
Structure parameters (Å and
degrees).
|
| |
|
|
|
|
C1 = MP2/cc-pV5Z optimization. |
|
Cs = B3P86/aug-cc-pVTZ optimization. |
|
|
|
|
|
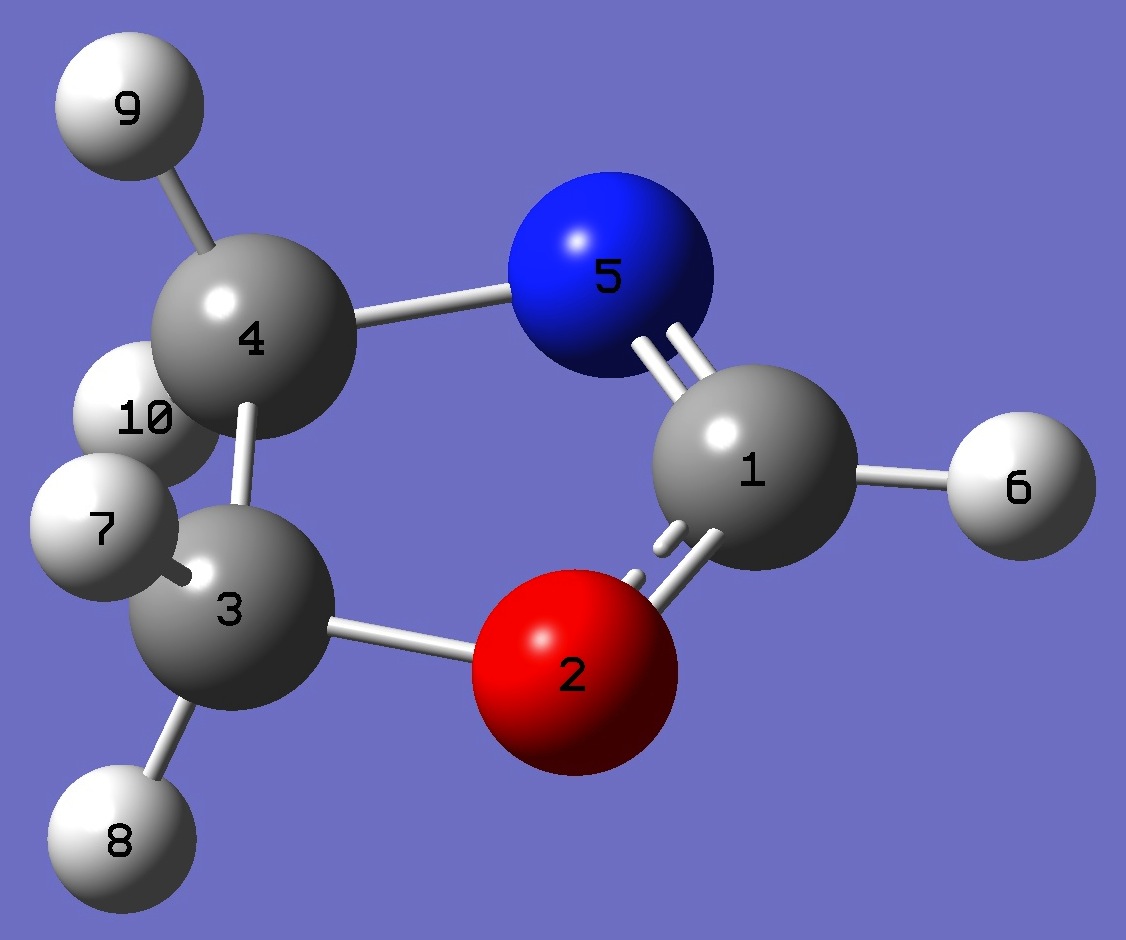
C
O,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
N,1,B4,2,A3,3,D2,0
H,1,B5,5,A4,4,D3,0
H,3,B6,2,A5,1,D4,0
H,3,B7,2,A6,1,D5,0
H,4,B8,3,A7,2,D6,0
H,4,B9,3,A8,2,D7,0
|
|
|
|
|
|
|
|
|
|
|
| C1 |
Cs |
|
|
|
|
|
B1=1.354
B2=1.448
B3=1.535
B4=1.472
B5=1.079
B6=1.087
B7=1.084
B8=1.086
B9=1.084
A1=104.8
A2=104.0
A3=104.8
A4=125.3
A5=107.6
A6=107.9
A7=112.6
A8=112.6
D1=8.9
D2=-9.9
D3=177.9
D4=-111.0
D5=130.5
D6=-129.3
D7=108.0
|
B1=1.34986267
B2=1.44530626
B3=1.53991491
B4=1.26032942
B5=1.08207032
B6=1.08888141
B7=1.08888141
B8=1.09066379
B9=1.09066379
A1=105.37177065
A2=103.86435901
A3=119.96470603
A4=125.44825138
A5=107.94037378
A6=107.94037378
A7=112.67994337
A8=112.67994337
D1=0.
D2=0.
D3=180.
D4=-121.0476441
D5=121.0476441
D6=-119.08384728
D7=119.08384728
|
|
|
|
|
|
|
|