|
| |
|
|
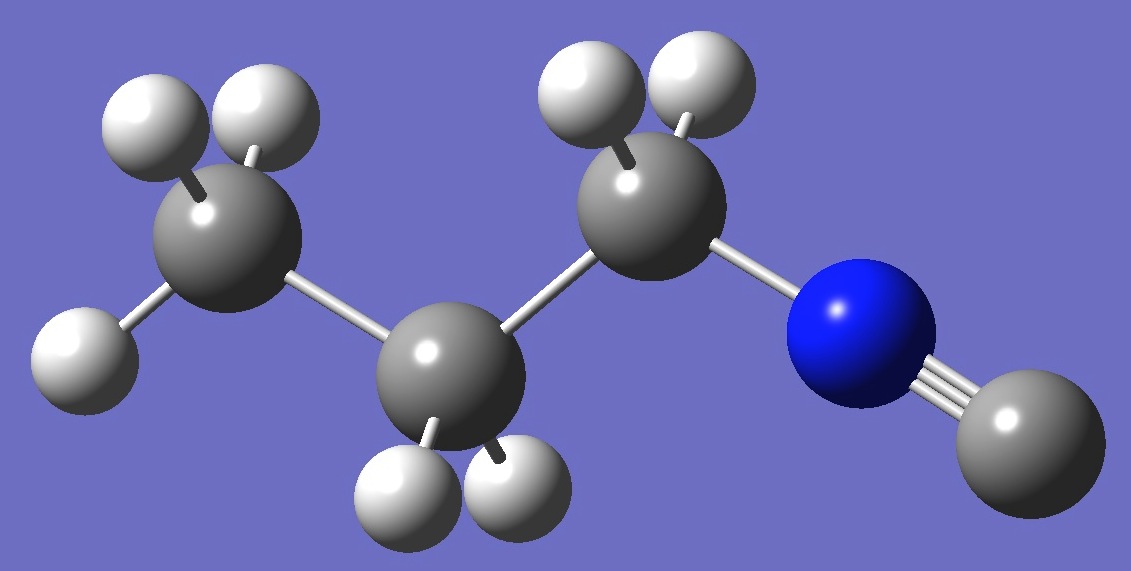
| Table 2. Molecular
structure parameters: B3P86/6-31G(3d,3p) and mPW1PW91/6-31G(3d,3p) opt structures (Å
and degrees). |
| |
|
|
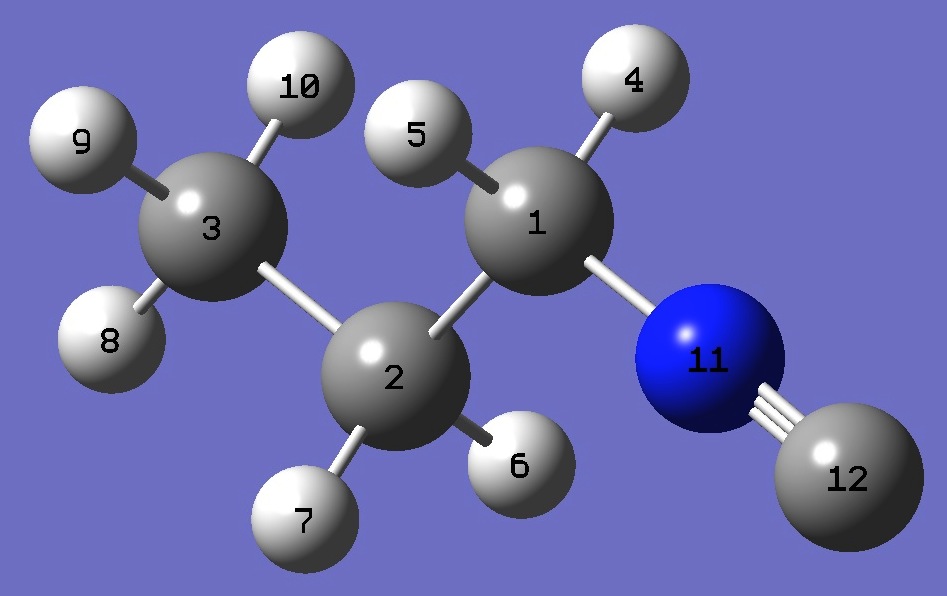
|
C
C,1,B1
C,2,B2,1,A1
H,1,B3,2,A2,3,D1,0
H,1,B4,2,A3,3,D2,0
H,2,B5,1,A4,3,D3,0
H,2,B6,1,A5,3,D4,0
H,3,B7,2,A6,1,D5,0
H,3,B8,2,A7,1,D6,0
H,3,B9,2,A8,1,D7,0
N,1,B10,2,A9,3,D8,0
C,11,B11,1,A10,2,D9,0
|
|
|
|
|
B3P86
|
mPW1PW91
|
|
|
|
|
B1=1.52583987
B2=1.52160464
B3=1.09505949
B4=1.09505949
B5=1.09407782
B6=1.09407782
B7=1.09186873
B8=1.09410273
B9=1.09410273
B10=1.41924139
B11=1.17094366
A1=111.44706368
A2=110.51054188
A3=110.51054188
A4=108.7534553
A5=108.7534553
A6=110.9126031
A7=111.44226211
A8=111.44226211
A9=112.05362125
A10=178.19636302
D1=-59.26786262
D2=59.26786262
D3=122.10805679
D4=-122.10805679
D5=180.
D6=-60.15959385
D7=60.15959385
D8=180.
D9=0.
|
B1=1.52580432
B2=1.52153395
B3=1.0941235
B4=1.0941235
B5=1.09334493
B6=1.09334493
B7=1.09117651
B8=1.09334516
B9=1.09334516
B10=1.41882012
B11=1.16940857
A1=111.51616687
A2=110.53308632
A3=110.53308632
A4=108.74226572
A5=108.74226572
A6=110.87387568
A7=111.45631272
A8=111.45631272
A9=112.006657
A10=178.2126864
D1=-59.30084843
D2=59.30084843
D3=122.11614645
D4=-122.11614645
D5=180.
D6=-60.18012561
D7=60.18012561
D8=180.
D9=0.
|
|
|
|
|
|