|
| |
|
|
|
|
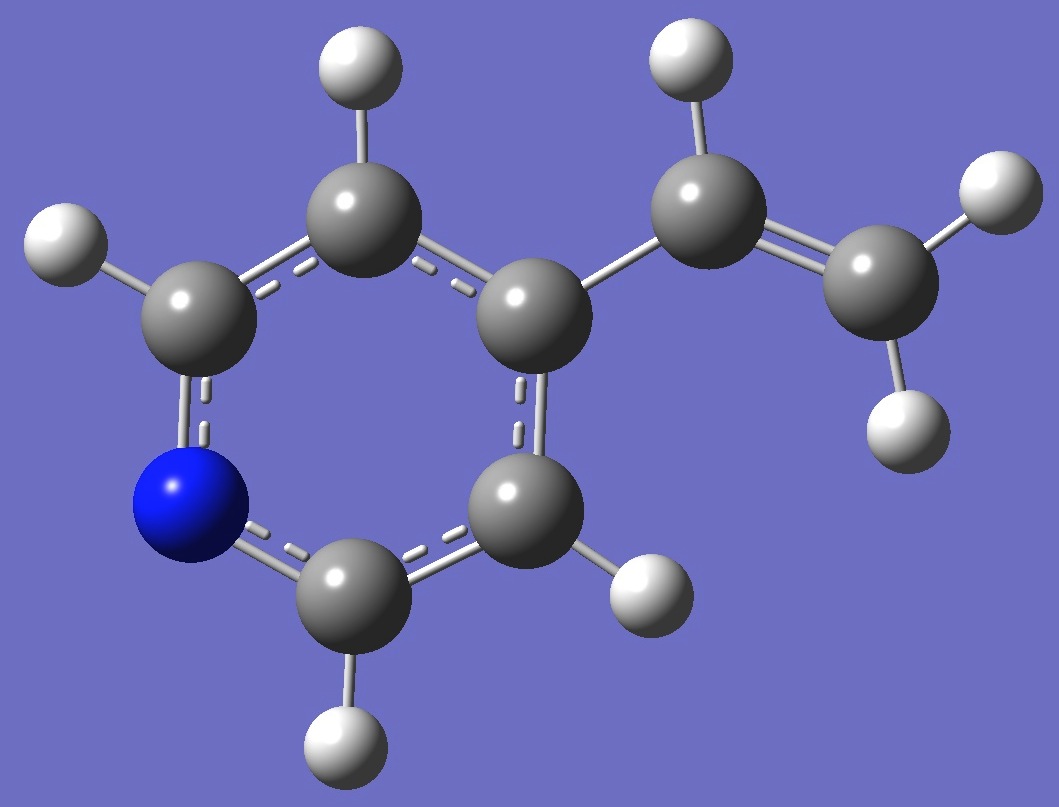
Table 2. 4-Vinylpyridine.
Molecular structure parameters, ropt (Å and
degrees).
|
|
|
|
|
|
|
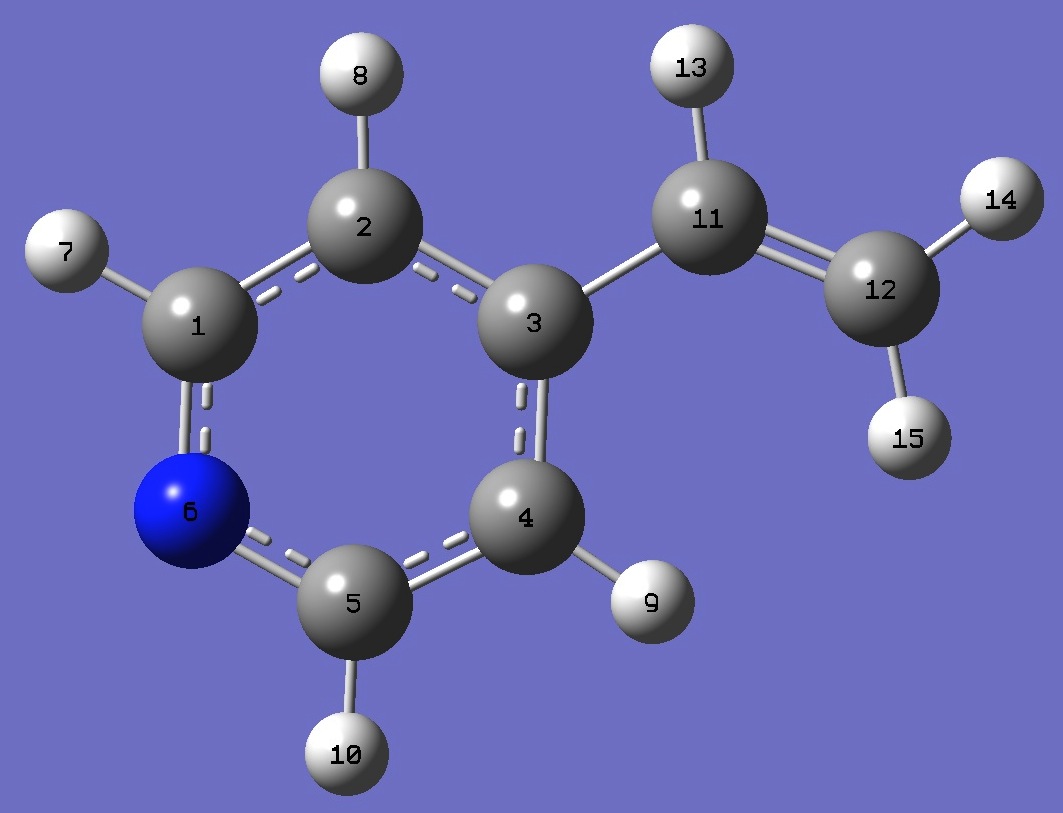
C
C,1,B1
C,2,B2,1,A1
C,3,B3,2,A2,1,D1,0
C,4,B4,3,A3,2,D2,0
N,1,B5,2,A4,3,D3,0
H,1,B6,6,A5,5,D4,0
H,2,B7,1,A6,6,D5,0
H,4,B8,3,A7,2,D6,0
H,5,B9,4,A8,3,D7,0
C,3,B10,2,A9,1,D8,0
C,11,B11,3,A10,2,D9,0
H,11,B12,3,A11,2,D10,0
H,12,B13,11,A12,3,D11,0
H,12,B14,11,A13,3,D12,0
|
|
|
|
|
|
|
|
|
|
|
B1=1.38872668
B2=1.39688009
B3=1.39862485
B4=1.38553664
B5=1.33216614
B6=1.08787175
B7=1.08555816
B8=1.08424036
B9=1.08775568
B10=1.46484814
B11=1.33329171
B12=1.08811877
B13=1.08394687
B14=1.08516398
|
A1=119.64659597
A2=116.50466077
A3=119.32825183
A4=123.87584893
A5=116.05712983
A6=120.04809535
A7=121.2385538
A8=119.89953899
A9=119.6321603
A10=126.57865505
A11=114.83702123
A12=120.91318034
A13=122.56574955
|
D1=0.
D2=0.
D3=0.
D4=180.
D5=180.
D6=180.
D7=180.
D8=180.
D9=180.
D10=0.
D11=180.
D12=0.
|
|
|
|
|
|
|
|