| |
|||||||
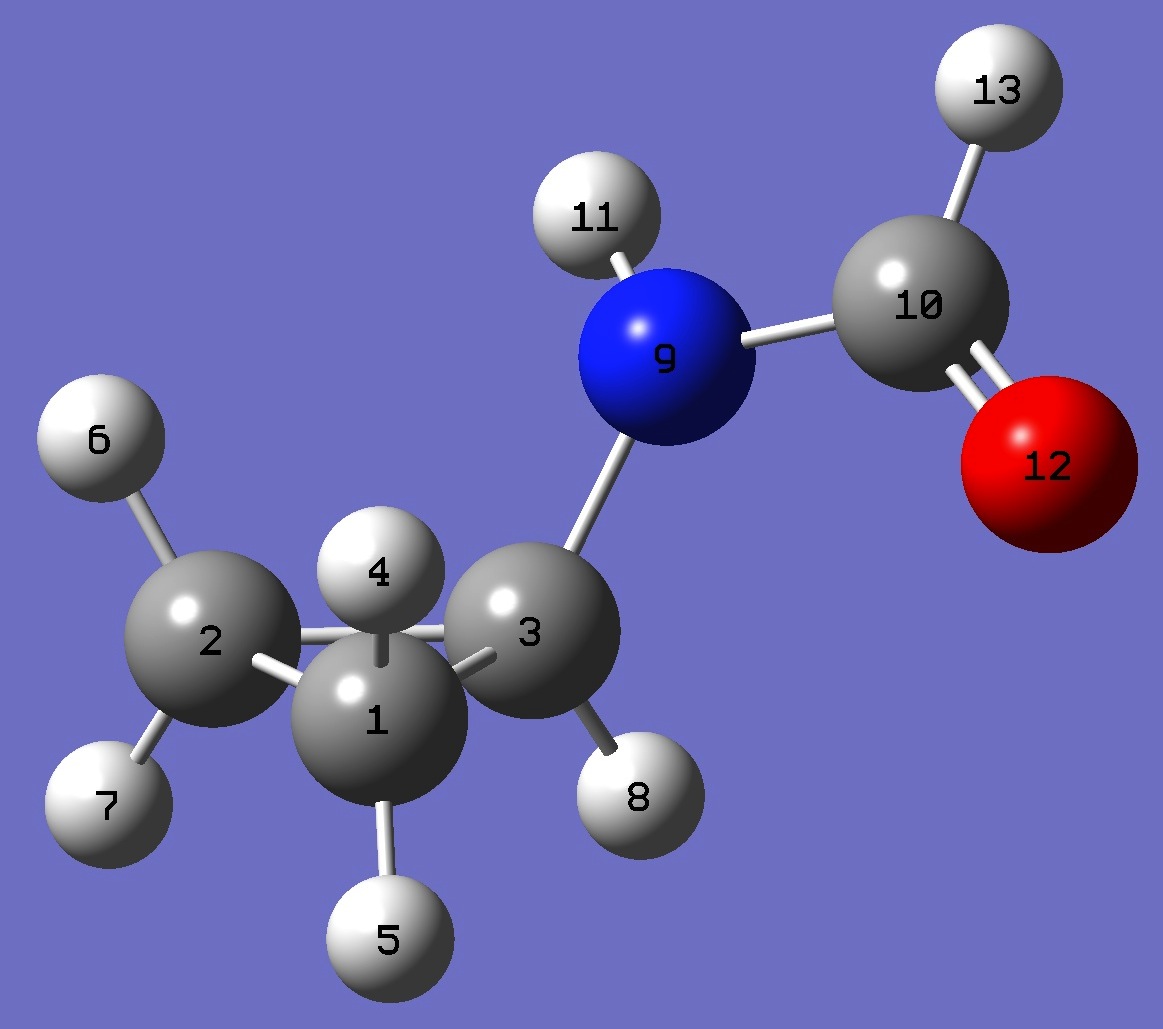
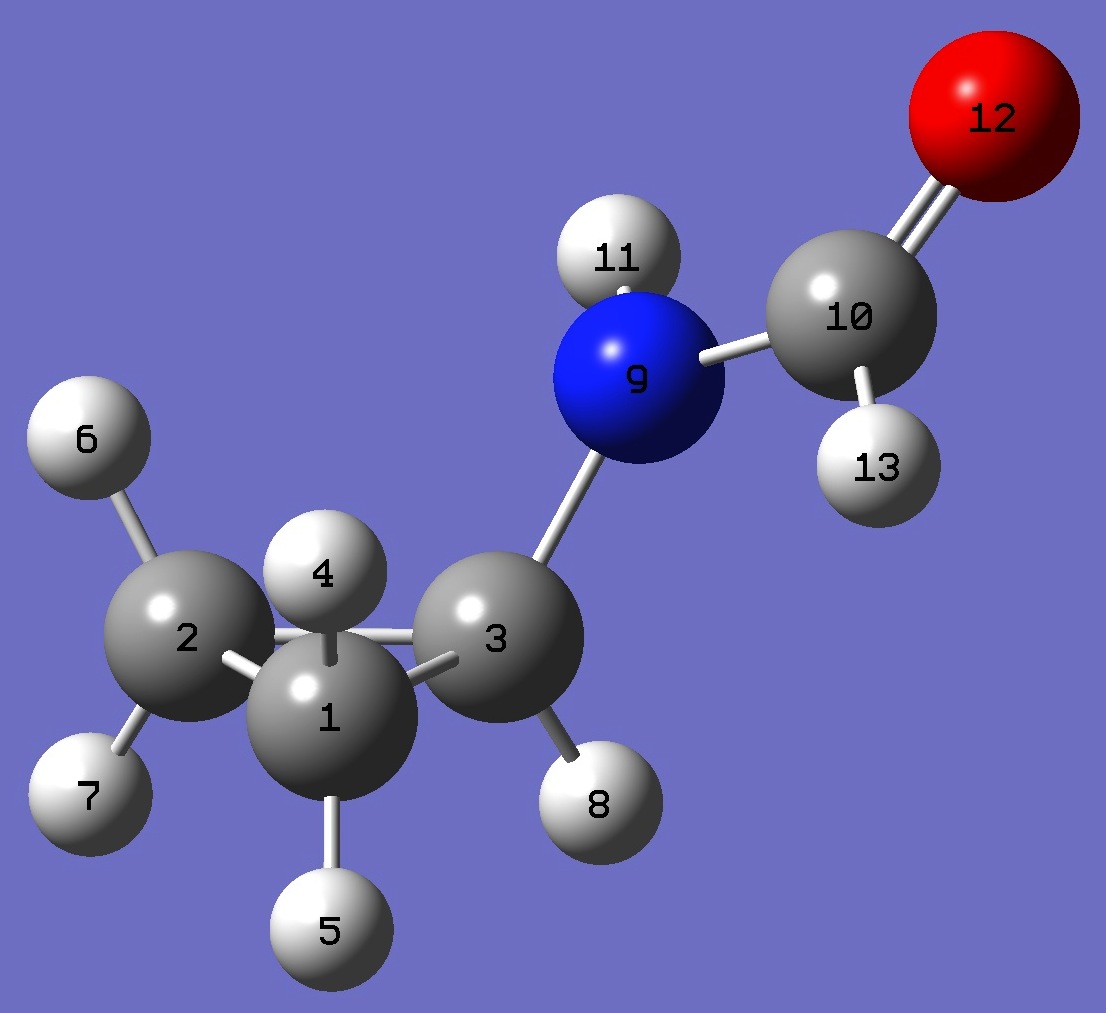
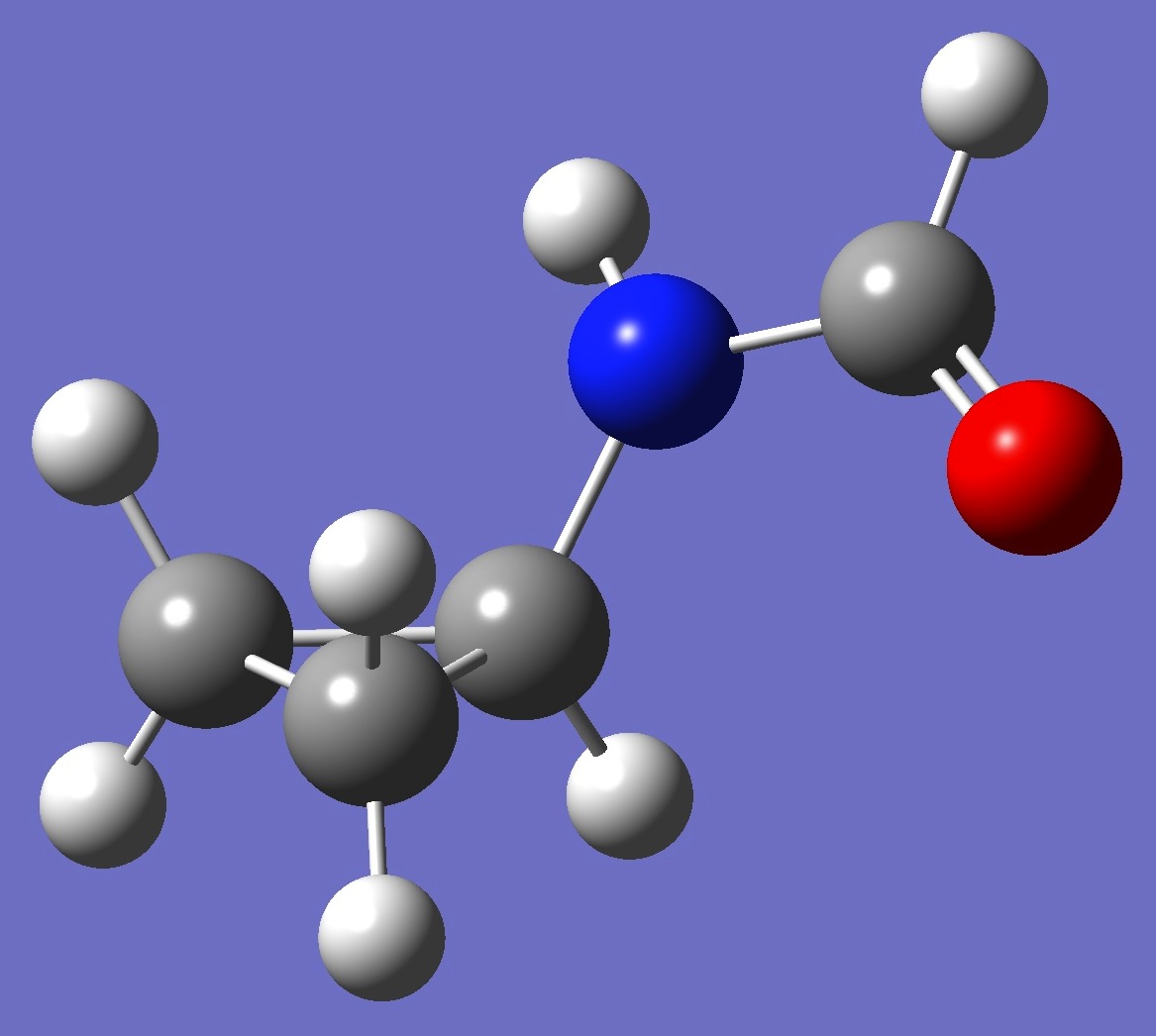
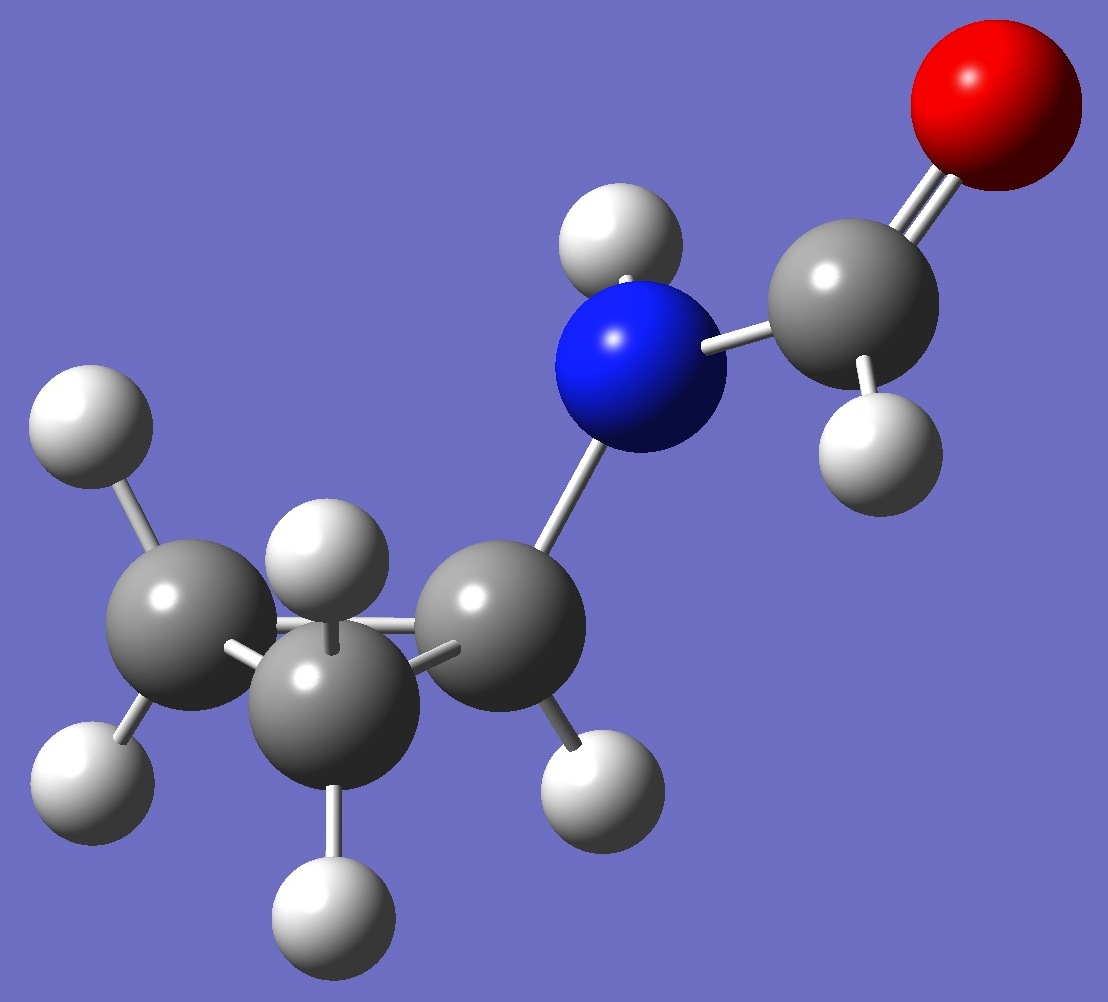
| Table 1. Nitrogen nqcc's in cis-N-cyclopropylformamide (MHz). Calculation was made on a molecular structure given by HF/6-311++G(3df,3pd) optimization. | |||||||
| |
|||||||
| Calc. |
Expt. |
||||||
| 14N | Xaa |
1.533 |
|||||
| Xbb | 1.673 |
||||||
| Xcc | - |
3.206 |
|||||
| Xab | 0.094 |
||||||
| Xac | - |
1.557 |
|||||
| Xbc | 1.390 |
||||||
| RSD |
0.030(1.3 %) | ||||||
| Xxx | 1.699 |
||||||
| Xyy | 2.299 |
||||||
| Xzz | - |
3.998 |
|||||
| |
|||||||
| |
|||||||
| Table 2. Nitrogen nqcc's in trans-N-cyclopropylformamide (MHz). Calculation was made on a molecular structure given by HF/6-311++G(3df,3pd) optimization. | |||||||
| |
|||||||
| Calc. |
Expt. |
||||||
| 14N | Xaa |
1.843 |
|||||
| Xbb | 1.577 |
||||||
| Xcc | - |
3.420 |
|||||
| Xab | 0.103 |
||||||
| Xac | - |
1.606 |
|||||
| Xbc | 1.167 |
||||||
| RSD |
0.030(1.3 %) | ||||||
| Xxx | 1.731 |
||||||
| Xyy | 2.374 |
||||||
| Xzz | - |
4.105 |
|||||
| |
|||||||
| |
||||
| Table 4. N-Cyclopropylformamide. Rotational Constants (MHz). Calc = HF/6-311++G(3df,3pd) ropt. | ||||
| Calc. |
Expt. [1] | |||
| cis | A |
9378. |
8999.0439(27) | |
| B |
2426. |
2450.13114(99) | ||
| C |
2094. |
2101.4817(10) | ||
| trans | A |
12820. |
12298.34(84) | |
| B |
1929. |
1917.2153(26) | ||
| C |
1806. |
1796.1162(25) | ||