|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
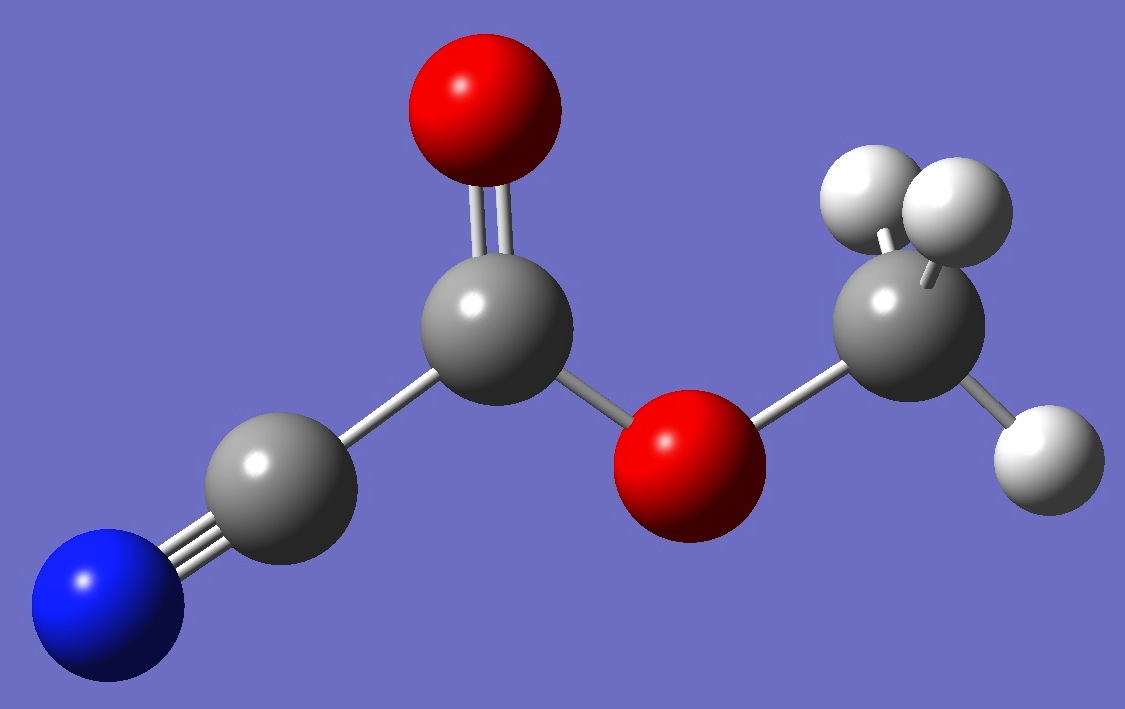
CH3OC(=O)CN
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in Methylcyanoformate |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
microwave spectrum of methylcyanoformate was assigned by Durig et al.
[1]. Quadrupole splitting, however, was unresolvable. An re molecular structure was derived by Groner and Warren [2].
|
|
|
Calculation of the nitrogen nqcc
tensor was made here on this re
structure. These calculated nqcc's are given in Table 1. Structure parameters are given in Table 2.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to
the principal axes of the inertia tensor, subscripts x,y,z to the
principal axes of the nqcc tensor. The nqcc y-axis is chosen
coincident with the inertia c-axis, these are perpendicular to the Cs
plane of the molecule. Ø (degrees) is the angle between
its subscripted parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
RSD is the residual standard
deviation
of calibration of the B3PW91/6-311+G(df,pd) model for calculation of
the efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1.
Nitrogen nqcc's in Methylcyanoformate (MHz). Calculation was made on re structure.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
4.258 |
|
|
|
|
|
Xbb |
|
1.451 |
|
|
|
|
|
Xcc |
|
2.807 |
|
|
|
|
|
|Xab| |
|
1.566 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.853 |
|
|
|
|
|
Xyy |
|
2.807 |
|
|
|
|
|
Xzz |
- |
4.660 |
|
|
|
|
|
ETA |
|
0.205 |
|
|
|
|
|
Øz,a |
|
14.38 |
|
|
|
|
|
Øa,CN |
|
14.27 |
|
|
|
|
|
Øz,CN |
|
0.10 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
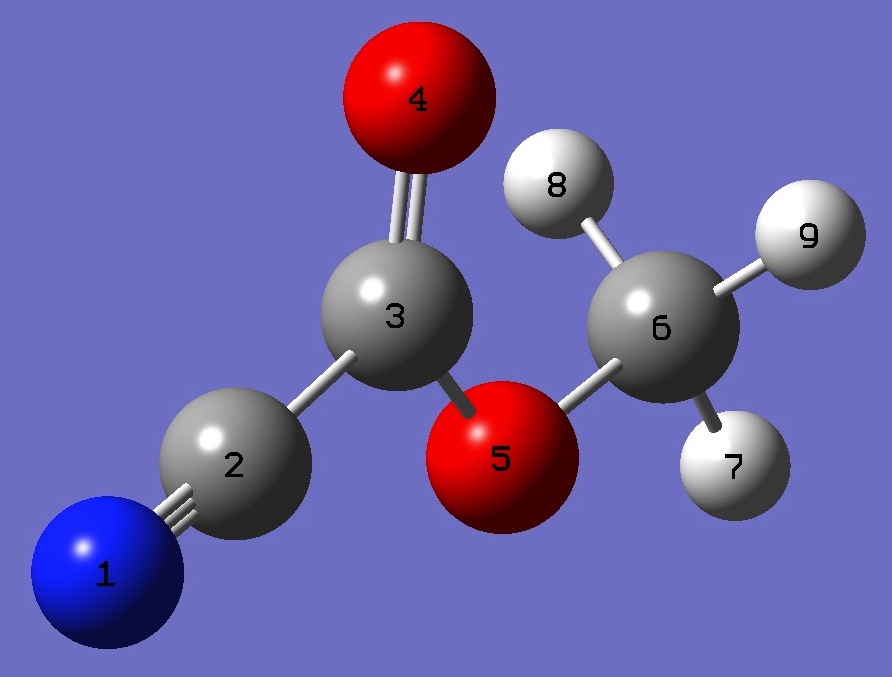
| Table 2. Methylcyanoformate, re [2] molecular structure parameters (Å and degrees). |
| |
|
|
|
|
| 
|
NC(2) |
|
1.1545(10) |
|
C(2)C(3) |
|
1.4807(86) |
|
C(3)O(4) |
|
1.1912(62) |
|
C(3)O(5) |
|
1.3192(45) |
|
O(5)C(6) |
|
1.4395(10) |
|
C(6)H(7)
|
|
1.0807(35) |
|
C(6)H(8,9) |
|
1.1020(72) |
|
C(3)C(2)N(1) |
|
177.60(25) |
|
O(5)C(3)O(4) |
|
128.12(87)
|
|
C(6)O(5)C(3) |
|
113.61(29)
|
|
H(7)C(6)O(5) |
|
105.28(17)
|
|
H(8,9)C(6)O(5) |
|
109.81(4)
|
|
H(8,9)C(6)O(5)H(9,8) |
±
|
120.81(62)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] J.R.Durig, P.Groner, and J.Lin, J.Chem.Phys. 96(11),8062(1992)
|
|
|
[2] P.Groner and R.D.Warren, J.Mol.Spectrosc. 599,323(2001).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3OC(=O)Cl |
HC(=O)CN
|
CH3C(=O)CN |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3OCOCN.html |
|
|
|
|
|
|
Last
Modified 29 Nov 2013
|
|
|
|
|
|
|
|
|
|
|

