|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCC-C(H)NH |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in Propargylimine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
microwave spectra of both Z- and E-isomers of propargylimine were
observed and assigned by Sugie et al. [1]. Rotational constants,
dipole moments, and 14N nuclear quadrupole coupling constants were determined.
|
|
|
|
|
|
|
|
|
|
|
|
|
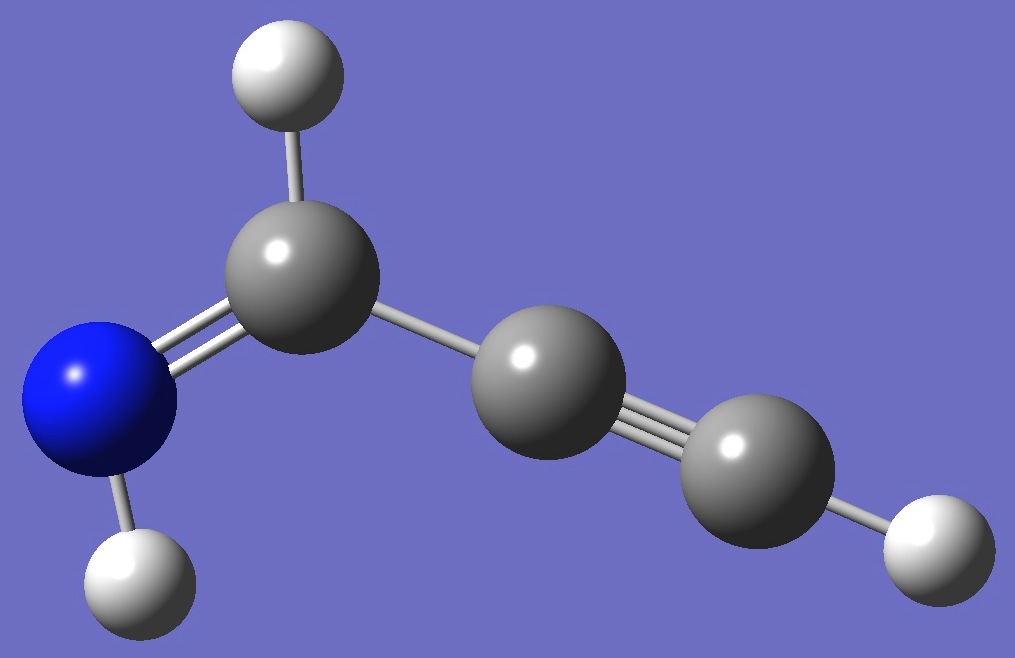
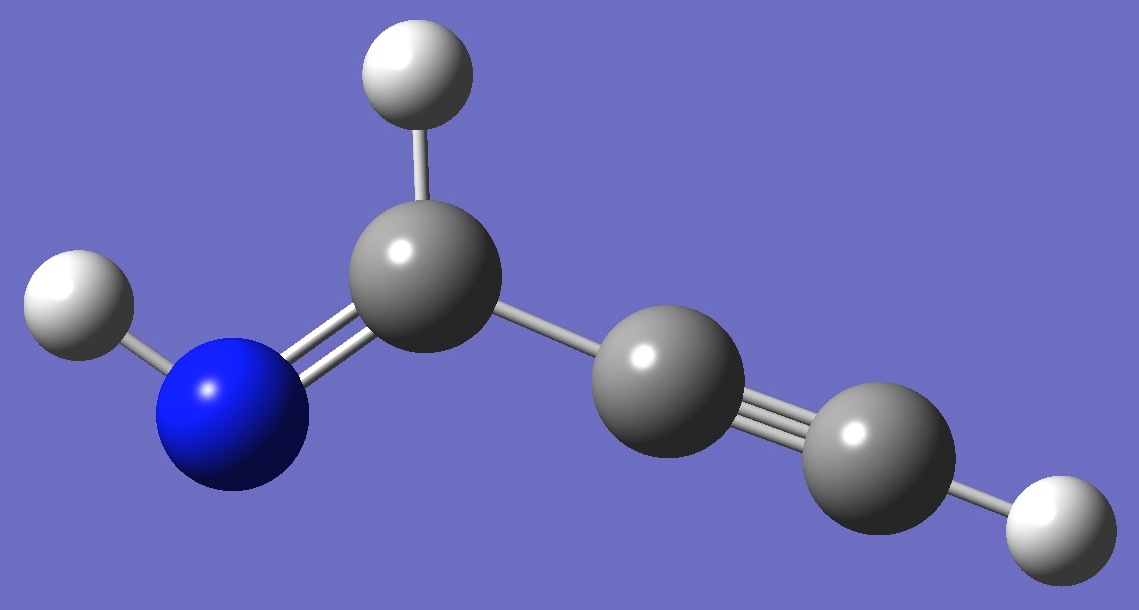
For each conformer, shown below, calculation of the nitrogen nqcc
tensor was made on a molecular structures given by MP2/6-311+G(3df,3pd) optimization with empirically corrected approximate equilibrium single and triple CC bond lengths and MP2/aug-cc-pVTZ optimization also with empirically corrected approximate equilibrium single and triple CC bond lengths. These
calculated nqcc's are given in Tables 1 and 2. Structure
parameters are given in Z-Matrix format in Table 3, rotational
constants and dipole moments in Table 4.
|
|
|
|
|
|
|
|
|
|
|
|
|
Z-Propargylimine
|
|
|
E-Propargylimine
|
|
|
|
At the
|
|
|
|
MP2/aug-cc-pVTZ |
|
|
level of theory,
|
|
|
EZ < EE
|
|
|
by 4.0 kJ/mol
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz.
Ø (degrees) is the angle between its subscripted parameters.
|
|
|
RSD is the calibration residual standard deviation of
the B3PW91/6-311+G(df,pd) model for calculation of the nitrogen efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 14N
nqcc tensor in Z-Propargylimine
(MHz). Calculation was made on molecular structures given by (1)
MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ optimization each with
approximate re single and triple CC bond lengths. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
-
|
4.057
|
-
|
4.047
|
-
|
4.1(2)
|
|
|
Xbb |
|
0.691
|
|
0.691
|
|
1.4(4)
|
|
|
Xcc |
|
3.366
|
|
3.357
|
|
2.7(3)
|
|
|
Xab
|
|
1.166
|
|
1.166
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.962
|
|
0.962
|
|
|
|
|
Xyy |
|
3.366 |
|
3.357 |
|
|
|
|
Xzz |
-
|
4.328
|
-
|
4.319
|
|
|
|
|
ETA |
|
0.555
|
|
0.554
|
|
|
|
|
Øz,a |
|
13.08
|
|
13.11
|
|
|
|
|
Øa,bi* |
|
14.87
|
|
14.82
|
|
|
|
|
Øz,bi |
|
1.79
|
|
1.72
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* "bi" is bisector of CNH angle.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 14N
nqcc tensor in E-Propargylimine
(MHz). Calculation was made on molecular structures given by (1)
MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ optimization, each with
approximate re single and triple CC bond lengths. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc (1) |
|
Calc (2)
|
|
Expt [1]
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
|
0.948
|
|
0.952
|
|
0.1(3)
|
|
|
Xbb |
-
|
4.311
|
-
|
4.306
|
-
|
3.8(2)
|
|
|
Xcc |
|
3.363
|
|
3.354
|
|
3.7(4)
|
|
|
Xab
|
|
0.485
|
|
0.492
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
0.993
|
|
0.997
|
|
|
|
|
Xyy |
|
3.363
|
|
3.354
|
|
|
|
|
Xzz |
-
|
4.356
|
-
|
4.352
|
|
|
|
|
ETA |
|
0.544
|
|
0.542
|
|
|
|
|
Øz,a |
|
95.23
|
|
95.30
|
|
|
|
|
Øa,bi* |
|
98.25
|
|
98.25
|
|
|
|
|
Øz,bi |
|
3.02
|
|
2.95
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* "bi" is bisector of CNH angle.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 3. Propargylimine: MP2/6-311+G(3df,3pd) and MP2/aug-cc-pVTZ optimized structure parameters (Å
and degrees). Approximate re bond lenths are given in parentheses. |
|
|
|
|
|
|
|
|
|
|
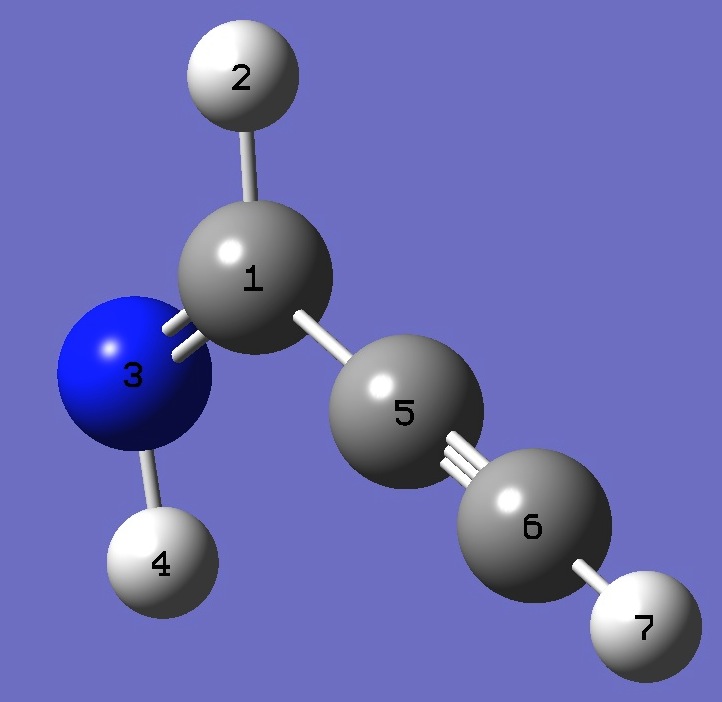
| Z-Propargylimine
|
|
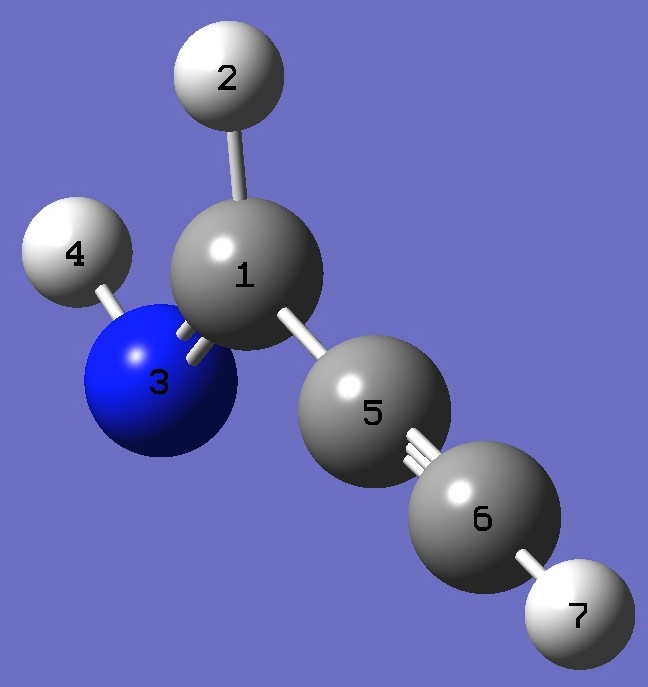
E-Propargylimine
|
|
C
H,1,B1
N,1,B2,2,A1
H,3,B3,1,A2,2,D1,0
C,1,B4,3,A3,4,D2,0
C,5,B5,3,A4,1,D3,0
H,6,B6,3,A5,1,D4,0
|
|
|
|
|
|
____________________ MP2/6-311+G(3df,3pd)____________________
|
|
|
|
|
|
|
|
|
|
|
|
B1=1.08657074
B2=1.28264734
B3=1.02138743
B4=1.43437427 (1.4365)
B5=1.21659694 (1.2064)
B6=1.06227896
A1=117.8016985
A2=109.55327158
A3=125.57321157
A4=154.54277151
A5=162.47988488
D1=180.
D2=0.
D3=180.
D4=180.
|
|
B1=1.09086173
B2=1.28225496
B3=1.01991455
B4=1.43238068 (1.4346)
B5=1.2156033 (1.2056)
B6=1.06207224
A1=123.45952937
A2=109.08136526
A3=120.98334695
A4=155.59596498
A5=162.26655693
D1=0.
D2=180.
D3=180.
D4=180.
|
|
|
|
|
|
|
|
|
|
|
| ______________________ MP2/aug-cc-pVTZ______________________ |
|
|
|
|
|
|
|
|
|
|
|
B1=1.0864415
B2=1.28409614
B3=1.02234058
B4=1.43428466 (1.4361)
B5=1.21776082 (1.2064)
B6=1.06245275
A1=117.70607867
A2=109.48606543
A3=125.62617892
A4=154.31826247
A5=162.58276506
D1=180.
D2=0.
D3=180.
D4=180.
|
|
B1=1.0905593
B2=1.28393075
B3=1.02091118
B4=1.43246001 (1.4344)
B5=1.2167906 (1.2056)
B6=1.06228171
A1=123.47663952
A2=109.07216717
A3=120.97968429
A4=155.48010454
A5=162.32618829
D1=0.
D2=180.
D3=180.
D4=180.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 4. Propargylimine: Rotational Constants (MHz) and Dipole Moments (D). Calc (1)
= MP2/6-311+G(3df,3pd) and Calc (2) = MP2/aug-cc-pVTZ optimization, each with
approximate re single and triple CC bond lengths.
|
|
|
|
|
|
|
|
|
__________Z-Propargylimine__________
|
|
|
|
Calc (1)
|
Calc (2)
|
Expt [1,2]
|
|
|
|
|
|
|
|
|
A |
54427
|
54279
|
54640.228(83) [1]
|
|
|
B |
4868
|
4868
|
4862.4191(59)
|
|
|
C |
4469
|
4467
|
4458.1986(52)
|
|
|
|µa|
|
2.40
|
2.40
|
2.145(2) [2]
|
|
|
|µb| |
0.21
|
0.21
|
0.182(6)
|
|
|
|
|
|
|
|
|
|
__________E-Propargylimine__________ |
|
|
|
Calc (1)
|
Calc (2)
|
Expt [1]
|
|
|
|
|
|
|
|
|
A
|
62765
|
62598
|
63099.320(79)
|
|
|
B
|
4772
|
4771
|
4766.6104(72)
|
|
|
C
|
4435
|
4433
|
4425.5144(64)
|
|
|
|µa| |
0.39
|
0.40
|
|
|
|
|µb| |
2.05
|
2.05
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] M.Sugie, H.Takeo, and C.Matsumura, J.Mol.Spectrosc. 111,83(1985).
|
|
|
[2] D.McNaughton, O.I.Osman, and H.W.Kroto, J.Mol.Struct. 190,195(1988).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Methylenimine
|
Ethanimine | Propenimine
|
|
|
|
|
Difluoromethanimine |
N-Methylmethanimine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
HCCCHNH.html |
|
|
|
|
|
|
Last
Modified 22 Jan 2014
|
|
|
|
|
|
|
|
|
|
|



