|
|
|
|
|
|
|
|
|
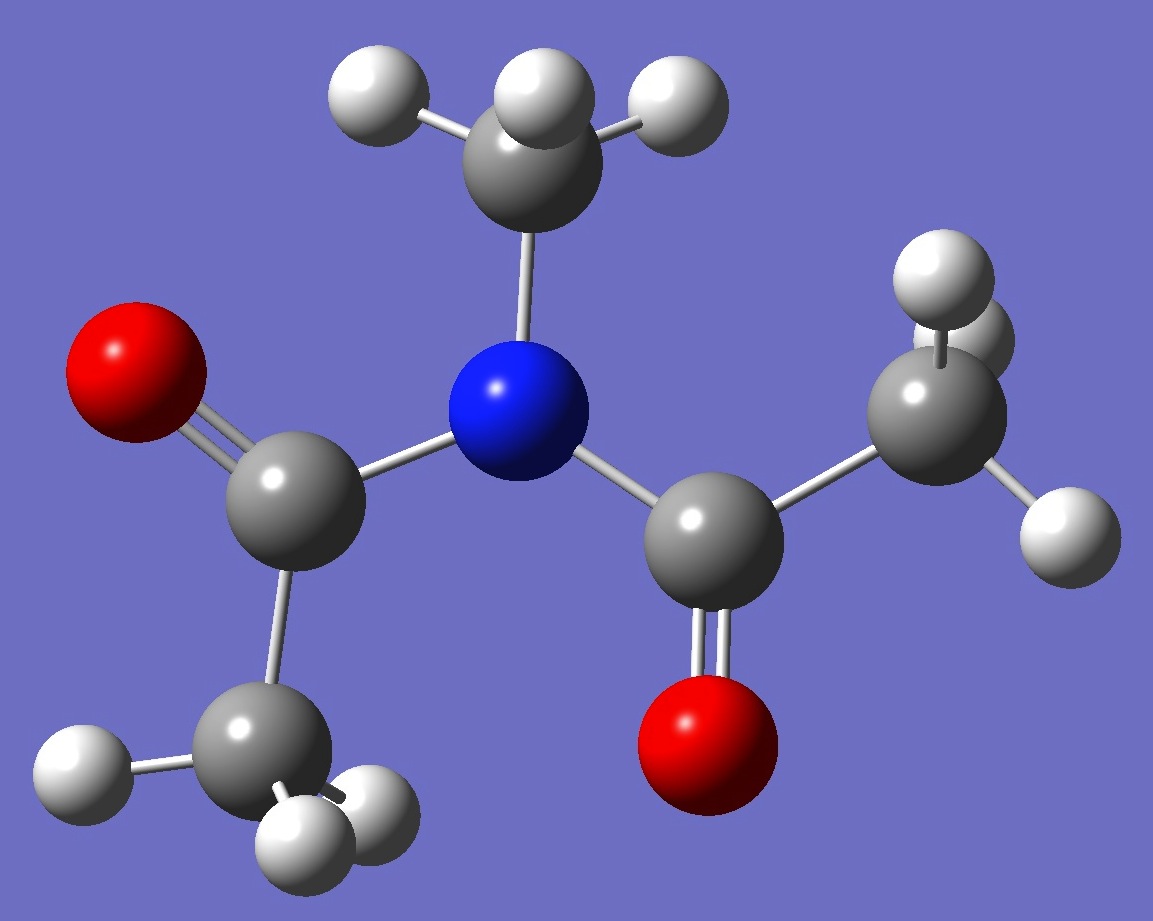
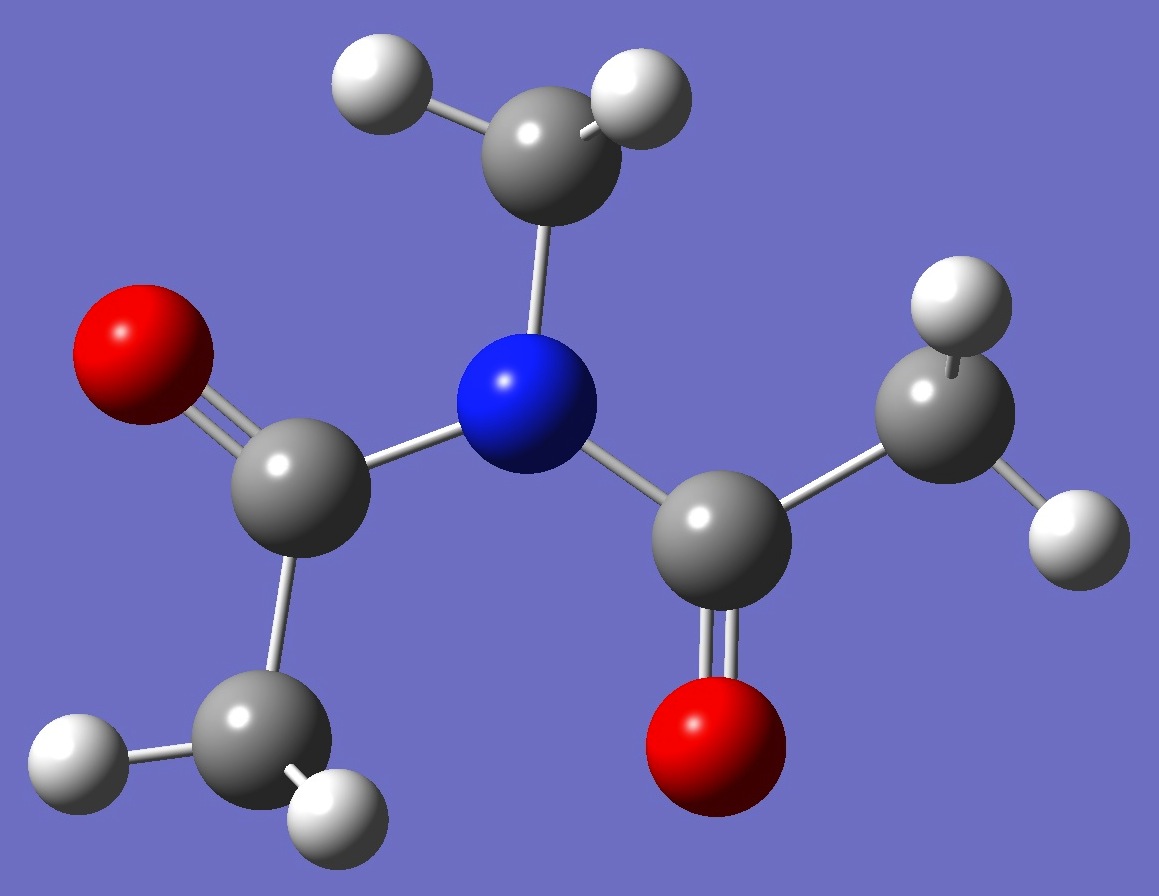
| Table 3.
N-Methyldiacetamide. MP2/6-311++G(d,p) Structure parameters (┼ and degrees).
|
|
|
|
|
|
|
|
|
|
|
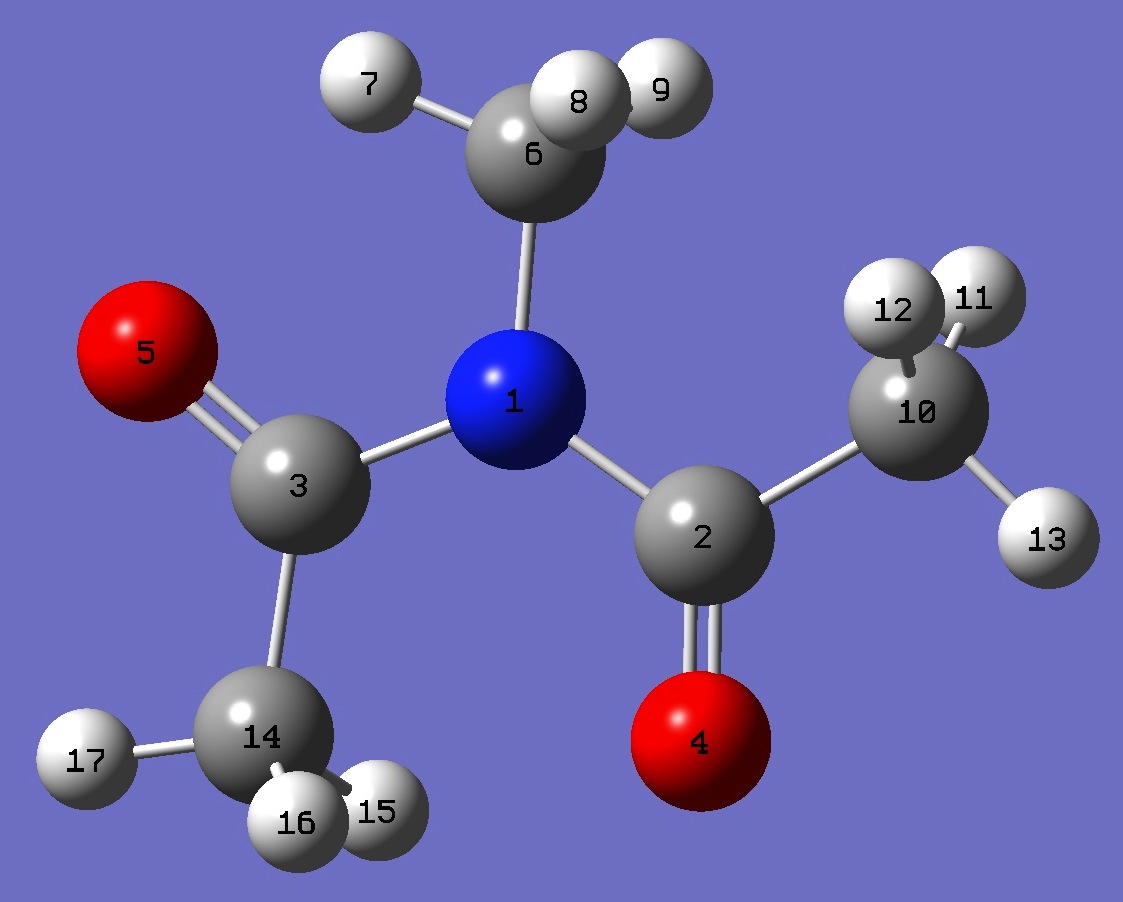
N
C,1,B1
C,1,B2,2,A1
O,2,B3,1,A2,3,D1,0
O,3,B4,1,A3,2,D2,0
C,1,B5,2,A4,4,D3,0
H,6,B6,1,A5,2,D4,0
H,6,B7,1,A6,2,D5,0
H,6,B8,1,A7,2,D6,0
C,2,B9,1,A8,3,D7,0
H,10,B10,2,A9,1,D8,0
H,10,B11,2,A10,1,D9,0
H,10,B12,2,A11,1,D10,0
C,3,B13,1,A12,2,D11,0
H,14,B14,3,A13,1,D12,0
H,14,B15,3,A14,1,D13,0
H,14,B16,3,A15,1,D14,0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cs Symmetry
|
C1 Symmetry
|
|
|
|
|
|
|
|
|
| B1=1.40687544
B2=1.42573562
B3=1.21954088
B4=1.21960555
B5=1.46868542
B6=1.08737397
B7=1.09179243
B8=1.09179243
B9=1.51712959
B10=1.09299745
B11=1.09299745
B12=1.08921446
B13=1.50791574
B14=1.09046133
B15=1.09046133
B16=1.09010462
A1=124.44064877
A2=123.54350539
A3=119.30844501
A4=119.42902783
A5=107.35726617
A6=110.86209419
A7=110.86209419
A8=115.38636853
A9=111.36139992
A10=111.36139992
A11=107.2453843
A12=119.34321107
A13=111.16054199
A14=111.16054199
A15=106.47052939
D1=0.
D2=180.
D3=180.
D4=180.
D5=-61.01131384
D6=61.01131384
D7=180.
D8=-60.2595961
D9=60.2595961
D10=180.
D11=0.
D12=-59.39154945
D13=59.39154945
D14=180.
|
B1=1.40556954
B2=1.42259903
B3=1.21984811
B4=1.21962035
B5=1.46813493
B6=1.08795677
B7=1.09442349
B8=1.08828618
B9=1.51675788
B10=1.09370186
B11=1.09298438
B12=1.08931537
B13=1.50807695
B14=1.09010761
B15=1.09102183
B16=1.09058816
A1=123.41544403
A2=123.01739987
A3=118.9317703
A4=120.18292313
A5=107.98699172
A6=111.47741872
A7=109.98076803
A8=115.83604469
A9=111.09627686
A10=111.60076893
A11=107.18320127
A12=119.0939365
A13=111.89739169
A14=110.31124851
A15=106.62570919
D1=0.79735294
D2=161.46684202
D3=167.56159794
D4=164.26734956
D5=-76.89490376
D6=45.09373615
D7=-179.43713048
D8=-62.07456254
D9=58.67625999
D10=178.50903261
D11=-21.41387913
D12=-41.54575375
D13=77.520002
D14=-163.07074755
|
|
|
|
|
|
|
|
|
|
|