|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
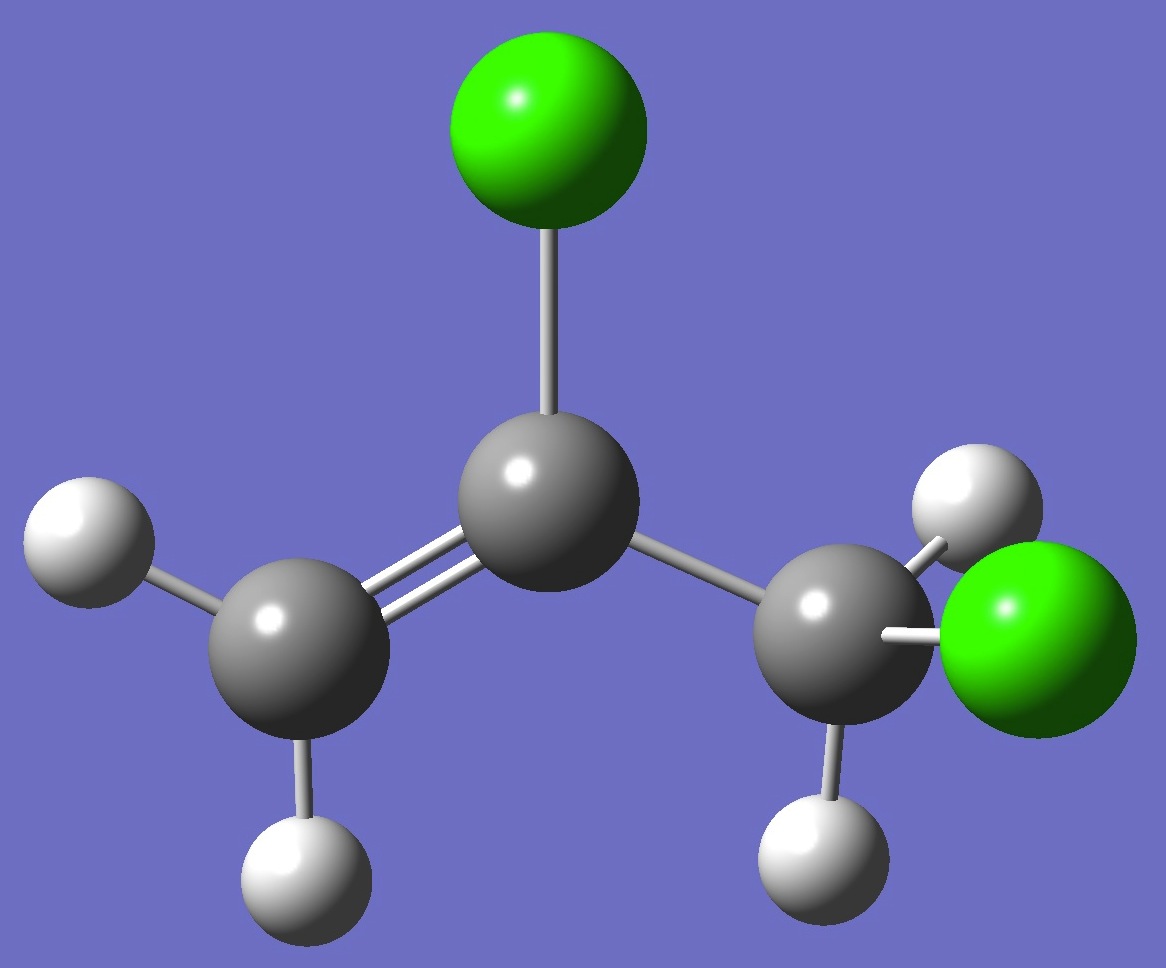
CH2=C(Cl)-CH2Cl
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in gauche-2,3-Dichloropropene
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine nqcc's
in anti- and gauche-2,3-dichloropropene were determined by Dikkumbura et al. [1]. The results for gauche are given on this page. See here for anti.
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the chlorine nqcc tensors
was made here on molecular structures given by MP2/aug-cc-pVTZ optimization (ropt) and on this
same structures but with empirically corrected bond lenghts for C-C, C=C,
and CCl (reemp). These calculated nqcc's are compared
with the experimental values in Tables 1 - 5. Structure
parameters are given in Table 6, rotational constants in Table 7. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 5,
subscripts a,b,c refer to the principal axes of the inertia
tensor; x,y,z to the principal axes of the nqcc
tensor. Ø (degrees) is the angle between its
subscripted parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean
square
difference between calculated and experimental diagonal nqcc's
(percentage of the average of the magnitudes of the experimental
nqcc's). RSD is the calibration residual standard deviation of
the B1LYP/TZV(3df,2p) model for calculation of the efg's/nqcc's, which may be
taken as an estimate of the uncertainty in the calculated nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NOTE: The experimental results given below in Tables 1 - 4 were derived here using Kisiel's program QDIAG.f and data given in Table 4.2 of Ref. [1]. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1. 35Cl(2)
nqcc's in CH2=C35Cl(2)-CH235Cl(3) (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calculation was made on the
MP2/aug-cc-pVTZ optimized molecular structure (ropt), and on this same
structure but with empirically corrected bond lenghts for C-C, C=C, and
CCl (reemp). |
|
|
|
|
|
|
|
|
|
|
|
|
|
ropt |
|
reemp |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa
|
|
16.15 |
|
16.05
|
|
15.695(2)
|
|
|
Xbb |
-
|
49.06 |
-
|
48.86
|
-
|
48.901(2)
|
|
|
Xcc |
|
32.92 |
|
32.81
|
|
33.206(2)
|
|
|
Xab |
-
|
44.10 |
-
|
44.14
|
|
43.68(8)
|
|
|
Xac |
|
0.23
|
|
0.26
|
|
0.47(15)
|
|
|
Xbc |
|
- 5.89
|
|
- 5.86
|
|
- 5.62(29)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.32 (1.0 %)
|
|
0.31 (1.0 %)
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
39.72 |
|
39.64
|
|
38.58(14)
|
|
|
Xyy |
|
31.92 |
|
31.80
|
|
32.60(12)
|
|
|
Xzz |
- |
71.64 |
-
|
71.44
|
-
|
71.19(7)
|
|
|
ETA |
-
|
0.109 |
-
|
0.110
|
-
|
0.0841(3)
|
|
|
Øz,CCl |
|
0.54 |
|
0.55
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2. 35Cl(3)
nqcc's in CH2=C35Cl(2)-CH235Cl(3) (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calculation was made on the
MP2/aug-cc-pVTZ optimized molecular structure (ropt), and on this same
structure but with empirically corrected bond lenghts for C-C, C=C, and
CCl (reemp). |
|
|
|
|
|
|
|
|
|
|
|
|
|
ropt |
|
reemp |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa
|
- |
15.36 |
-
|
15.29
|
-
|
15.504(2)
|
|
|
Xbb |
|
12.29 |
|
12.25
|
|
12.302(3)
|
|
|
Xcc |
|
3.07
|
|
3.04
|
|
3.202(3)
|
|
|
Xab |
|
36.81 |
|
36.75
|
-
|
36.79(11)
|
|
|
Xac |
|
42.21
|
|
42.13
|
-
|
41.80(10)
|
|
|
Xbc |
-
|
30.54
|
-
|
30.51
|
-
|
30.07(13)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.11 (1.1 %)
|
|
0.16 (1.5 %)
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
36.82 |
|
36.75
|
|
36.58(11)
|
|
|
Xyy |
|
38.61 |
|
38.55
|
|
38.30(12)
|
|
|
Xzz |
- |
75.43 |
- |
75.30
|
-
|
74.88(12)
|
|
|
ETA |
|
0.0237 |
|
0.0238
|
|
0.0230(21)
|
|
|
Øz,CCl |
|
0.60 |
|
0.61
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 3. Cl
nqcc's in CH2=C35Cl(2)-CH237Cl(3) (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calculation was made on the
MP2/aug-cc-pVTZ optimized molecular structure (ropt), and on this same
structure but with empirically corrected bond lenghts for C-C, C=C, and
CCl (reemp). |
|
|
|
|
|
|
|
|
|
|
|
|
|
ropt |
|
reemp |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa (35Cl)
|
|
16.70
|
|
16.61
|
|
16.251(3)
|
|
|
Xbb |
-
|
49.64
|
-
|
49.44
|
-
|
49.462(3)
|
|
|
Xcc |
|
32.94
|
|
32.83
|
|
33.211(3)
|
|
|
Xab |
-
|
43.77
|
-
|
43.70
|
|
43.35(6)
|
|
|
Xac |
|
0.27
|
|
0.30
|
|
0.12(22)
|
|
|
Xbc |
|
- 5.93
|
-
|
- 5.83
|
|
- 4.7(5)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.32 (1.0 %)
|
|
0.30 (0.9 %)
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa (37Cl) |
-
|
12.72
|
-
|
12.66
|
-
|
12.812(4)
|
|
|
Xbb |
|
9.99
|
|
9.95
|
|
9.984(4)
|
|
|
Xcc |
|
2.73
|
|
2.71
|
|
2.828(4)
|
|
|
Xab |
|
29.01
|
|
28.96
|
-
|
28.93(9)
|
|
|
Xac |
|
33.32
|
|
33.26
|
-
|
33.39(14)
|
|
|
Xbc |
-
|
23.76
|
-
|
23.74
|
-
|
22.89(26)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.08 (0.9 %)
|
|
0.11 (1.3 %)
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 4. Cl
nqcc's in CH2=C37Cl(2)-CH235Cl(3) (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calculation was made on the
MP2/aug-cc-pVTZ optimized molecular structure (ropt), and on this same
structure but with empirically corrected bond lenghts for C-C, C=C, and
CCl (reemp). |
|
|
|
|
|
|
|
|
|
|
|
|
|
ropt |
|
reemp |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
|
Xaa (37Cl)
|
|
11.46
|
|
11.39
|
|
11.135(5)
|
|
|
Xbb |
-
|
37.40
|
-
|
37.24
|
-
|
37.300(4)
|
|
|
Xcc |
|
25.94
|
|
25.85
|
|
26.165(4)
|
|
|
Xab |
-
|
35.74
|
-
|
35.68
|
|
35.52(18)
|
|
|
Xac |
|
0.09
|
|
0.11
|
|
0.15(28)
|
|
|
Xbc |
|
- 4.66
|
|
- 4.64
|
|
- 3.8(4)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.23 (0.9 %)
|
|
0.23 (0.9 %)
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa (35Cl) |
-
|
14.04
|
-
|
13.97
|
-
|
14.183(5)
|
|
|
Xbb |
|
10.97
|
|
10.93
|
|
11.000(5)
|
|
|
Xcc |
|
3.07
|
|
3.04
|
|
3.182(5)
|
|
|
Xab |
|
37.28
|
|
37.22
|
-
|
37.19(22)
|
|
|
Xac |
|
41.66
|
|
41.58
|
-
|
41.60(21)
|
|
|
Xbc |
-
|
31.28
|
-
|
31.26
|
-
|
30.73(26)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.11 (1.1 %)
|
|
0.15 (1.6 %)
|
|
|
|
|
RSD |
|
0.49 (1.1 %) |
|
0.49 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 5. 37Cl
nqcc's in CH2=C37Cl(2)-CH237Cl(3) (MHz). |
|
|
|
|
|
|
|
|
|
|
|
Calculation was made on the
MP2/aug-cc-pVTZ optimized molecular structure (ropt), and on this same
structure but with empirically corrected bond lenghts for C-C, C=C, and
CCl (reemp). |
|
|
|
|
|
|
|
|
|
|
|
|
|
ropt |
|
reemp |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa Cl(2)
|
|
11.93
|
|
11.86
|
|
|
|
|
Xbb |
-
|
37.89
|
-
|
37.73
|
|
|
|
|
Xcc |
|
25.96
|
|
25.87
|
|
|
|
|
Xab |
-
|
35.39
|
-
|
35.33
|
|
|
|
|
Xac |
|
0.12
|
|
0.15
|
|
|
|
|
Xbc |
|
- 4.69
|
|
- 4.67
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
|
|
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa Cl(3) |
-
|
11.70
|
-
|
11.64
|
|
|
|
|
Xbb |
|
8.97
|
|
8.94
|
|
|
|
|
Xcc |
|
2.73
|
|
2.71
|
|
|
|
|
Xab |
|
29.38
|
|
29.33
|
|
|
|
|
Xac |
|
32.90
|
|
32.84
|
|
|
|
|
Xbc |
-
|
24.34
|
-
|
24.32
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
|
|
|
|
|
|
|
RSD |
|
0.44 (1.1 %) |
|
0.44 (1.1 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 6. gauche-2,3-Dichloropropene. MP2/aug-cc-pVTZ optimized molecular structure parameters, ropt
(Å and degrees). Corrected reemp C-C, C=C, and
CCl bond lengths are given in parentheses. |
| |
|
|
|
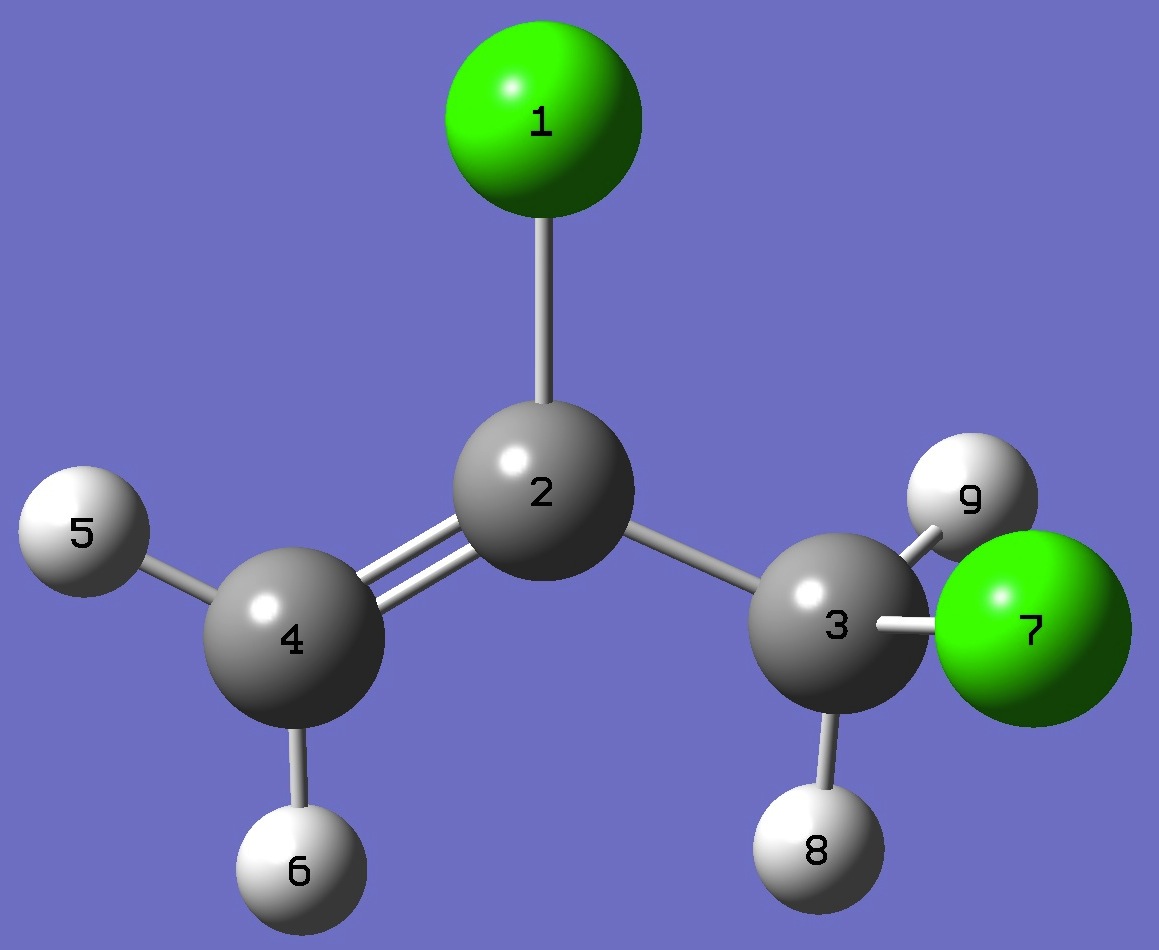
|
Cl
C,1,B1
C,2,B2,1,A1
C,2,B3,1,A2,3,D1,0
H,4,B4,2,A3,1,D2,0
H,4,B5,2,A4,1,D3,0
Cl,3,B6,2,A5,1,D4,0
H,3,B7,2,A6,1,D5,0
H,3,B8,2,A7,1,D6,0
|
|
B1=1.7335261 (1.7303)
B2=1.48760118 (1.4870)
B3=1.33397217 (1.3302)
B4=1.07914092
B5=1.08073511
B6=1.7895188 (1.7862)
B7=1.08655845
B8=1.08717158
A1=114.90265408
A2=121.11421322
A3=121.56103784
A4=119.24459342
A5=111.28304336
A6=110.03534485
A7=111.22622248
D1=-179.18506291
D2=0.32604627
D3=-179.49359143
D4=67.73380961
D5=-173.41129714
D6=-50.93853622
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 7. gauche-2,3-Dichloropropene.
Rotational constants (MHz). 35,35 species. |
| |
|
|
|
|
|
|
ropt |
reemp |
Expt. [1] |
|
|
|
|
|
|
A |
4819.
|
4838.
|
4825.77581(18)
|
|
B |
2044.
|
2049.
|
2027.99973(16)
|
|
C |
1570.
|
1574.
|
1559.15872(16)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] A.S.Dikkumbura, Masters
Theses, Eastern Illinois University, 2016. Faculty Advisor: R.A.Peebles. Available for
download at http://thekeep.elu.edu/theses/2443
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
anti-2,3-Dichloropropene
|
2-Chloropropene
| cis-3-Chloropropene
|
|
|
gauche-3-Chloropropene |
Chloropropenes
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
23DCPropene_G.html |
|
|
|
|
|
|
Last
Modified 15 July 2016 |
|
|
|
|
|
|
|
|
|
|

