| |
||||||||
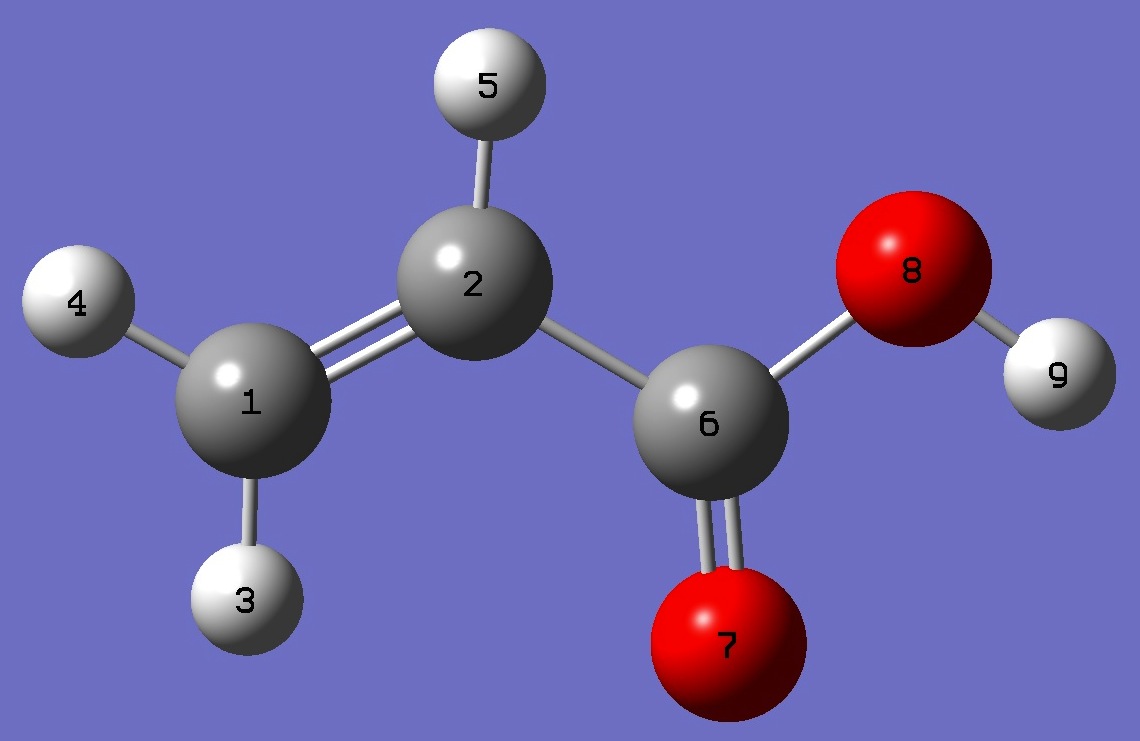
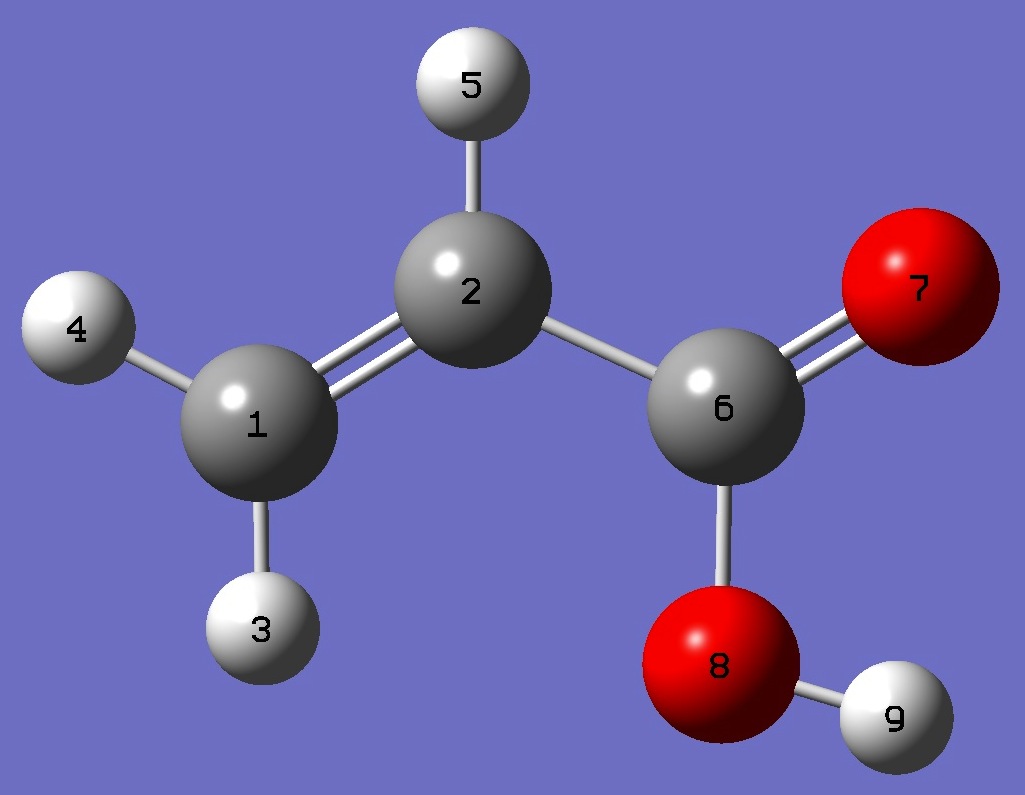
| Table 1. D nqcc's in s-cis-CH2=C(H)-C(=O)OD (kHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt molecular structures. | ||||||||
| |
||||||||
| Calc (1) | Calc (2) |
Expt [1] | ||||||
| |
||||||||
| Xaa | 150.9 | 148.2 | 148(6) |
|||||
| Xbb | 0.035 |
- 0.3 |
4(10) * |
|||||
| Xcc | - |
150.9 | - |
147.9 | - |
152(10) * |
||
| |Xab| | 182.6 |
178.6 |
||||||
| RMS | 2.9 (2.9 %) |
3.4 (3.4 %) |
||||||
| RSD | 1.1 (0.86 %) |
1.1 (0.86 %) | ||||||
| Xxx | - |
122.1 | - |
119.4 | ||||
| Xyy | - |
150.9 | - |
147.9 | ||||
| Xzz | 273.0 | 267.3 | ||||||
| ETA | 0.105 | 0.106 | ||||||
| Øz,a |
33.78 |
33.71 |
||||||
| Øa,OD | 35.23 |
35.17 |
||||||
| Øz,OD | 1.45 |
1.46 |
||||||
| |
||||||||
| |
||||||||
| Table 2. D nqcc's in s-trans-CH2=C(H)-C(=O)OD (kHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ ropt molecular structures. | ||||||||
| |
||||||||
| Calc (1) | Calc (2) |
Expt [1] | ||||||
| |
||||||||
| Xaa | 247.5 | 242.1 | 237(5) |
|||||
| Xbb | - 95.4 |
- 92.9 |
- 93(6) * |
|||||
| Xcc | - |
152.1 | - |
149.2 | - |
144(6) * |
||
| |Xab| | 99.4 |
97.8 |
||||||
| RMS | 7.8 (4.9 %) |
4.2 (2.7 %) |
||||||
| RSD | 1.1 (0.86 %) |
1.1 (0.86 %) | ||||||
| Xxx | - |
122.1 | - |
119.4 | ||||
| Xyy | - |
152.1 | - |
149.2 | ||||
| Xzz | 274.2 | 268.6 | ||||||
| ETA | 0.110 | 0.111 | ||||||
| Øz,a |
15.05 |
15.14 |
||||||
| Øa,CD | 13.54 |
13.63 |
||||||
| Øz,CD | 1.50 |
1.51 |
||||||
| |
||||||||
| Table 4. CH2=C(H)-C(=O)OD. Rotational Constants (MHz). ropt(1) = MP2/6-311+G(3df,3pd), ropt(2) = MP2/aug-cc-pVTZ optimized structures. | |||||
| ropt(1) | ropt(2) | Expt [1] | |||
| s-cis | A |
11062.3 |
11007.8 |
11068.1926(29) |
|
| B |
4100.1 |
4096.8 |
4075.5354(12) |
||
| C |
2991.4 |
2985.6 |
2979.5056(10) |
||
| |
|||||
| s-trans |
A |
10304.0 |
10263.0 |
10242.1235(20) |
|
| B |
4324.3 |
4317.6 |
4307.8658(16) |
||
| C |
3046.0 |
3039.2 |
3033.3858(10) |
||