|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quinoxaline
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in
Quinoxaline |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen nqcc tensors in quinoxaline
were calculated on a molecular structure optimized at the
B3P86/6-31G(3d,3p) level of theory (ropt). These
calculated nqcc's are compared with the experimental values of
McNaughton et al. [1] in Table 1. Structure parameters are
given in Table 2, rotational constants and electric dipole moments in
Table 3. |
|
|
|
|
|
|
|
|
|
|
|
|
In Table 1, subscripts a,b,c refer to
principal axes of the inertia tensor, subscripts x,y,z to principal
axes of the nqcc tensor. The nqcc y-axis is chosen coincident
with the inertia c-axis, these are perpendicular to the plane of the
molecule. Ø (degrees) is the angle between its subscripted
parameters. ETA = (Xxx - Xyy)/Xzz. |
|
|
RMS is the root mean square
difference between calculated and experimental nqcc's (percentage of
average experimental nqcc). RSD is the residual stand deviation
of calibration of the B3PW91/6-311+G(df,pd) model for calculation of
the nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen
nqcc's in Quinoxaline (MHz). Calculation was made
on the B3P86/6-31G(3d,3p) ropt structure. |
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
14N(1,2) |
Xaa |
|
1.203 |
|
1.2123(61) |
|
|
|
Xbb |
- |
4.973 |
- |
4.9402 * |
|
|
|
Xcc |
|
3.770 |
|
3.7279 * |
|
|
|
Xab |
± |
0.272 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.032 (0.96 %) |
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.215 |
|
|
|
|
|
Xyy |
|
3.770 |
|
|
|
|
|
Xzz |
- |
4.985 |
|
|
|
|
|
ETA |
|
0.512 |
|
|
|
|
|
Øz,b |
|
2.51 |
|
|
|
|
|
Øb,bi |
|
0.75
|
|
|
|
|
|
Øz,bi** |
|
3.27
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa and
Xbb - Xcc = -8.6681(64) MHz. |
|
|
** The z-axis makes an angle of
3.27
with the external bisector ('bi') of the CNC angle and tilts toward
C(3,4). See Table 2 for atomic numbering. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
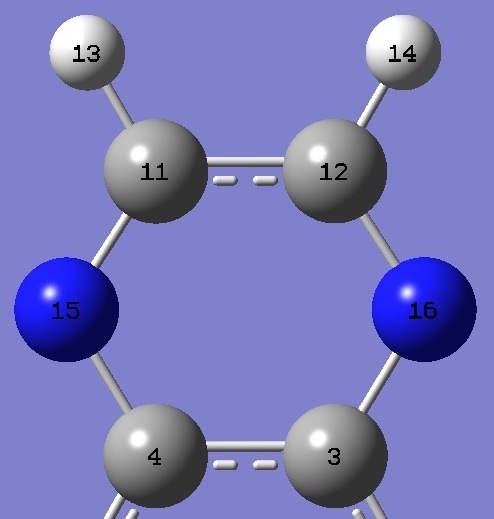
| Table 2. Quinoxaline and
Pyrazine. Selected molecular structure parameters,
B3P86/6-31G(3d,3p) ropt (Å and degrees).
The complete structure of quinoxaline is given here in Z-matrix format. |
| |
|
|
|
|
 |
|
|
Quinoxaline |
Pyrazine |
|
|
|
|
|
C(4)N |
1.3592 |
1.3317 |
|
NC(11) |
1.3099 |
1.3317 |
|
C(4)NC(11) |
116.14 |
115.77 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
| Table 3.
Quinoxaline, ropt.
Rotational constants (MHz) and B3PW91/6-311+G(df,pd) calculated
dipole moments (D). |
| |
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
|
|
|
|
|
|
A |
|
3187.98 |
|
3165.90728(64) |
|
B |
|
1319.30 |
|
1310.636904(72) |
|
C |
|
933.14 |
|
927.130503(46) |
|
|
|
|
|
|
|
µa |
|
0.56 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] D.McNaughton, P.D.Godfrey,
M.K.Jahn, D.A.Dewald, and J.-U.Grabow, J.Chem.Phys. 134,154305(2011). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pyrazine |
Quinazoline |
Phthalazine |
1,10-Phenanthroline |
|
|
Acridine |
Quinoline |
Isoquinoline |
Phenanthridine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quinoxaline.html |
|
|
|
|
|
|
Last
Modified 20 April 2011 |
|
|
|
|
|
|
|
|
|
|

