|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2-CN-C6H4OH
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in
ortho-Cyanophenol |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 14N
nqcc tensor in cis and trans conformers of o-cyanophenol
was made here
on molecular structures given by B3P86/6-31G(3d,3p) optimization
(assuming Cs symmetry). These calculated nqcc's are
given in Tables 1 and 2. Structure parameters are given in Table
3,
rotational
constants in Table 4. |
|
|
|
|
|
|
|
|
|
|
|
|
|
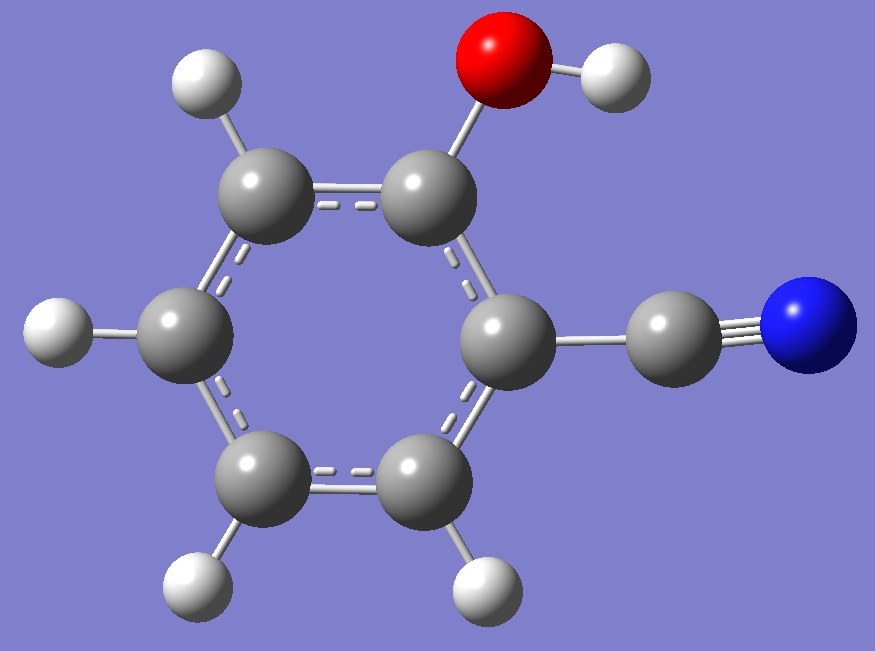
cis |
|
|
|
|
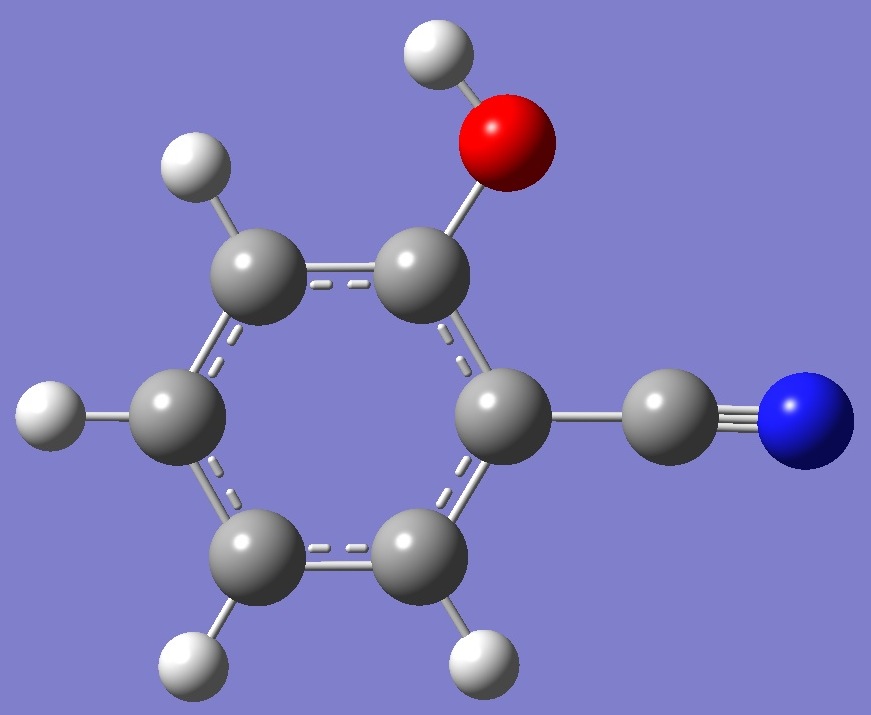
trans |
|
|
|
|
|
|
|
|
|
|
|
|
|
Ecis < Etrans |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 and 2, subscripts a,b,c
refer to the principal axes of the inertia tensor; x,y,z to the
principal axes of the nqcc tensor. The nqcc y-axis is chosen
coincident with the inertia c-axis. Ø (degrees) is the
angle between its
subscripted parameters. ETA = (Xxx - Xyy)/Xzz.
|
|
|
RMS is the root mean square
difference between calculated and
experimental diagonal nqcc's (percent of average of absolute
experimental diagonal nqcc's). RSD
is the calibration residual standard deviation of the
B3PW91/6-311+G(df,pd) model for
calculation of the nqcc's, which may be taken as an estimate of the
uncertainty in the calculated nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen nqcc's in cis-o-Cyanophenol (MHz). Calculation
was made on the B3P86/6-31G(3d,3p) ropt structure. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. [1] |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
4.208 |
- |
4.213(4) |
|
|
|
Xbb |
|
2.654 |
|
2.53(2) |
|
|
|
Xcc |
|
1.554 |
|
1.68(1) |
|
|
|
|Xac| |
|
0.428 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.105 (3.8 %) |
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.680 |
|
|
|
|
|
Xyy |
|
1.554 |
|
|
|
|
|
Xzz |
- |
4.235 |
|
|
|
|
|
ETA |
- |
0.266 |
|
|
|
|
|
Øz,a |
|
3.56 |
|
|
|
|
|
Øa,CN |
|
2.54 |
|
|
|
|
|
Øz,CN |
|
1.02 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen nqcc's
in trans-o-Cyanophenol
(MHz). Calculation
was made on the B3P86/6-31G(3d,3p) ropt structure. |
|
|
|
|
|
|
|
|
|
|
|
|
|
Calc. |
|
Expt. |
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
- |
4.112 |
|
|
|
|
|
Xbb |
|
2.220 |
|
|
|
|
|
Xcc |
|
1.892 |
|
|
|
|
|
|Xac| |
|
0.852 |
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.333 |
|
|
|
|
|
Xyy |
|
1.892 |
|
|
|
|
|
Xzz |
- |
4.225 |
|
|
|
|
|
ETA |
- |
0.104 |
|
|
|
|
|
Øz,a |
|
7.53 |
|
|
|
|
|
Øa,CN |
|
7.49 |
|
|
|
|
|
Øz,CN |
|
0.04 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
| Table 3. o-Cyanophenol.
Selected structure parameters, ropt (Å and
degrees). Complete structures are given here in Z-matrix format. |
| |
|
|
|
|
| |
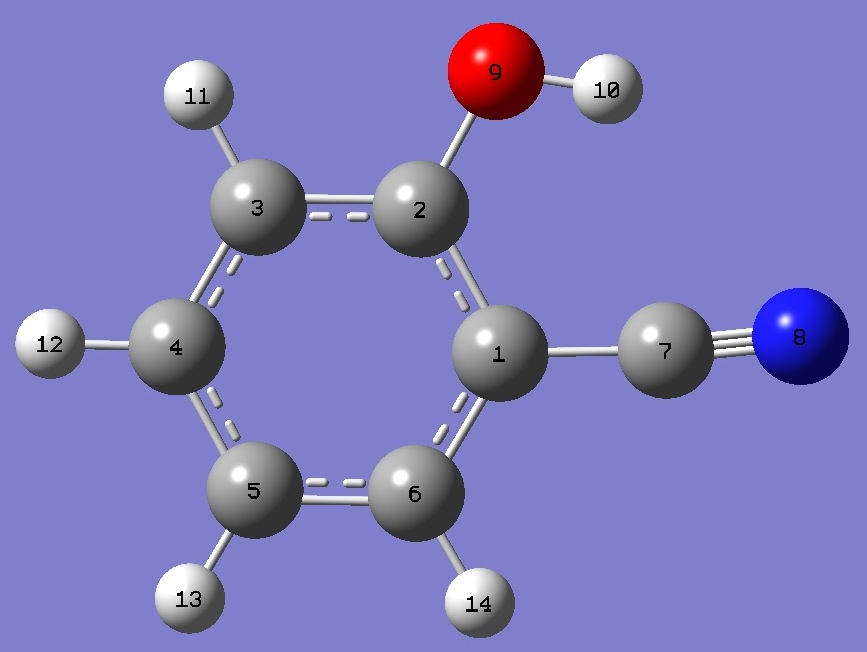
|
cis-o-Cyanophenol |
|
|
|
|
C(1)C(7) |
1.4222 |
|
C(7)N |
1.1603 |
|
C(1)C(7)N |
175.25 * |
|
C(2)C(1)C(6) |
120.18 |
|
|
|
|
C(2)O |
1.3450 |
|
OH |
0.9677 |
|
C(2)OH |
109.85 |
|
C(1)C(2)C(3) |
119.30 |
|
|
|
|
|
|
|
|
* N tilts toward OH |
|
|
|
|
|
|
|
|
|
|
|
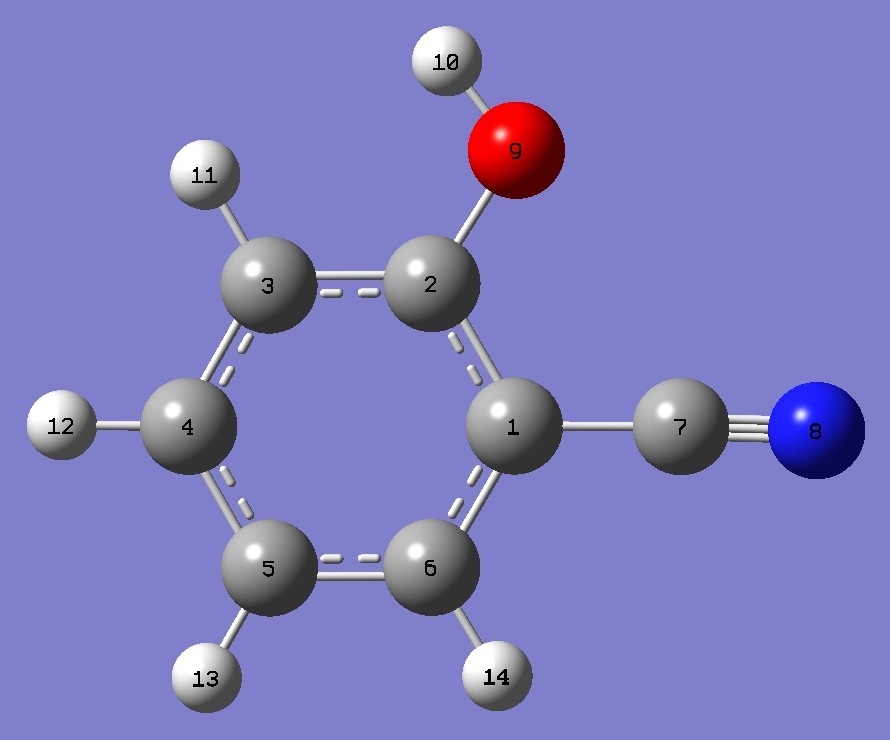
|
trans-o-Cyanophenol |
|
|
|
|
C(1)C(7) |
1.4252 |
|
C(7)N |
1.1582 |
|
C(1)C(7)N |
178.44 ** |
|
C(2)C(1)C(6) |
119.55 |
|
|
|
|
C(2)O |
1.3487 |
|
OH |
0.9625 |
|
C(2)OH |
109.59 |
|
C(1)C(2)C(3) |
119.50 |
|
|
|
|
|
|
|
|
** N tilts away from O |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
| Table 4. o-Cyanophenol.
Rotational Constants (MHz). |
| |
|
|
|
|
cis |
Calc. ropt |
Expt. [1] |
|
|
|
|
|
A |
3083.4 |
3053.758(2) |
|
B |
1518.7 |
1511.2760(3) |
|
C |
1017.5 |
1010.7989(2) |
|
|
|
|
|
trans |
Calc. ropt |
Expt. |
|
|
|
|
|
A |
2995.8 |
|
|
B |
1523.4 |
|
|
C |
1009.9 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] A.R.Conrad, N.Z.Barefoot, and
M.J.Tubergen, PCCP 12,8350(2011); 64th Ohio State University Symposium on
Molecular Spectroscopy, June 22-26, 2009. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Benzonitrile |
o-Tolunitrile |
o-Fluorobenzonitrile |
|
m-Cyanophenol |
m-Tolunitrile |
m-Fluorobenzonitrile |
|
p-Cyanophenol |
p-Tolunitrile |
p-Fluorobenzonitrile |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents
|
|
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
o_phenolCN.html |
|
|
|
|
|
|
Last
Modified 7 July 2009 |
|
|
|
|
|
|
|
|
|
|



