|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
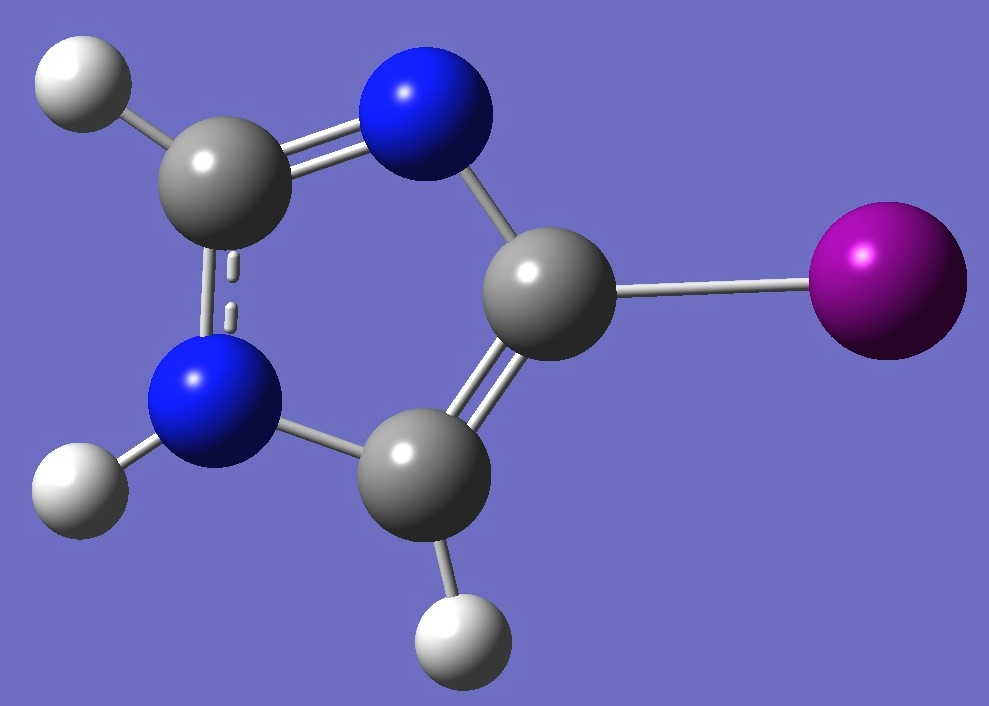
C3N2H3I |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iodine and Nitrogen
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
|
in 4-Iodoimidazole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Iodine and nitrogen nuclear quadrupole coiupling constants in 4-iodoimidazole were determined by Cooper et al. [1].
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 127I and 14N
nqcc's
was made here on a molecular structures given by B3LYP/cc-pVTZ
(cc-pVTZ-PP for I) optimization. Calculated nqcc's are compared
with the
experimental values in Tables 1 - 3. Structure parameters are
given in
Table 4, rotational constants in Table 5.
|
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 3, subscripts a,b,c refer to the principal axes of the inertia
tensor; x,y,z to the principal axes of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscripted parameters. RMS is the root mean
square difference between calculated and experimental diagonal nqcc's
(percentage of the average of the magnitudes of the experimental
nqcc's). RSD is the calibration residual standard deviation of
the B1LYP/TZV(df,p) model for calculation of iodine efg's/nqcc's; and in Tables 2 and 3, of the B3PW91/6-311+G(df,pd) model for nitrogen.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table
1. Iodine nqcc's in 4-Iodoimidazole (MHz). Calculation was
made on B3LYP/cc-pVTZ optimized
molecular structure.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc
|
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
127I |
Xaa |
-
|
1979.5
|
-
|
1980.759(10)
|
|
|
|
Xbb - cc
|
|
49.6
|
|
37.339(14)
|
|
|
|
Xbb |
|
1014.5
|
|
1009.049(9) *
|
|
|
|
Xcc |
|
965.0
|
|
971.710(9) *
|
|
|
|
|Xab| |
|
35.2
|
|
37.79(14)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
5.1 (0.38 %)
|
|
|
|
|
|
RSD |
|
15.2 (1.23 %)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1014.9
|
|
1009.5263(95)
|
|
|
|
Xyy |
|
965.0 |
|
971.7099(88)
|
|
|
|
Xzz |
-
|
1979.9
|
-
|
1981.236(11)
|
|
|
|
ETA
|
-
|
0.0252
|
|
|
|
|
|
Øz,a |
|
0.67
|
|
|
|
|
|
Øa,CI |
|
0.47
|
|
|
|
|
|
Øz,CI |
|
0.20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa and Xbb - cc
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 2. Nitrogen (pyrrolic) nqcc's in 4-Iodoimidazole (MHz). Calculation was
made on B3LYP/cc-pVTZ optimized
molecular structure.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc
|
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
14N(1) |
Xaa |
|
1.378
|
|
1.371(13)
|
|
|
|
Xbb - cc
|
|
3.608
|
|
3.674(11)
|
|
|
|
Xbb |
|
1.115
|
|
1.151(8) *
|
|
|
|
Xcc |
-
|
2.493
|
-
|
2.522(8) *
|
|
|
|
|Xab| |
|
0.063
|
|
-----
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.027 (1.6 %)
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
1.101
|
|
|
|
|
|
Xyy |
|
1.392
|
|
|
|
|
|
Xzz |
-
|
2.493 |
-
|
2.5223(86)
|
|
|
|
ETA
|
|
0.117
|
|
|
|
|
|
Øz,c |
|
0
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa and Xbb - cc |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 3. Nitrogen (pyridinic) nqcc's in 4-Iodoimidazole (MHz). Calculation was
made on B3LYP/cc-pVTZ optimized
molecular structure.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc
|
|
Expt [1] |
|
| |
|
|
|
|
|
|
|
|
14N(3) |
Xaa |
|
1.209
|
|
1.159(13)
|
|
|
|
Xbb - cc
|
-
|
5.296
|
-
|
5.169(11)
|
|
|
|
Xbb |
-
|
3.253
|
-
|
3.164(8) *
|
|
|
|
Xcc |
|
2.044
|
|
2.005(8) *
|
|
|
|
|Xab| |
|
2.111
|
|
1.71(37)
|
|
|
|
|
|
|
|
|
|
|
|
RMS |
|
0.063 (3.0 %)
|
|
|
|
|
|
RSD |
|
0.030 (1.3 %) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.044
|
|
1.75(23)
|
|
|
|
Xyy |
|
2.050
|
|
2.0051(86)
|
|
|
|
Xzz |
-
|
4.093
|
-
|
3.76(23)
|
|
|
|
ETA
|
|
0.00148
|
|
|
|
|
|
Øz,a |
|
68.29
|
|
|
|
|
|
Øa,bi** |
|
69.99
|
|
|
|
|
|
Øz,bi |
|
1.70
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* Calculated here from experimental Xaa and Xbb - cc
|
|
|
** "bi" is bisector of CNC angle.
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
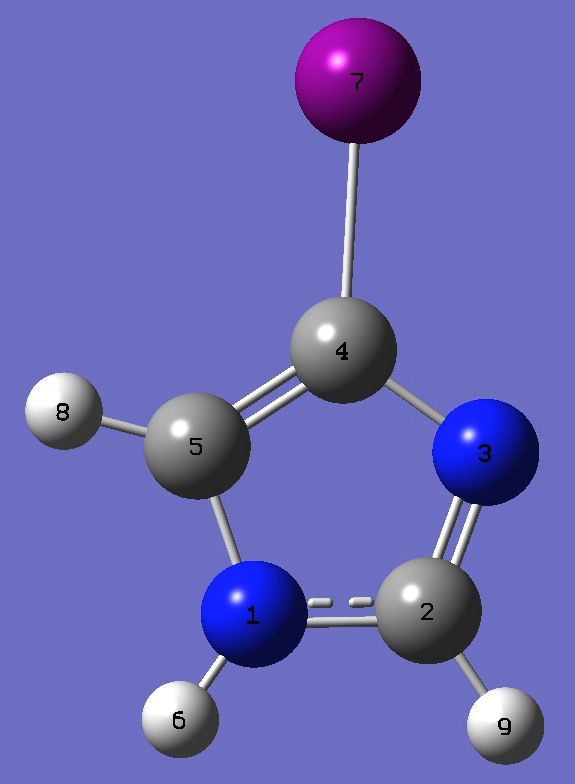
| Table 4. 4-Iodoimidazole: B3LYP/cc-pVTZ optimized structure parameters (Å and degrees). |
| |
|
|
|
|
N
C,1,R1
N,2,R2,1,A1
C,3,R3,2,A2,1,D1,0
C,4,R4,3,A3,2,D2,0
H,1,R5,2,A4,3,D3,0
I,4,R6,3,A5,2,D4,0
H,5,R7,4,A6,3,D5,0
H,2,R8,1,A7,5,D6,0
|
|
|
|
|
|
|
|
R1=1.36026216
R2=1.31260285
R3=1.36320614
R4=1.36845676
R5=1.00537868
R6=2.09385731
R7=1.07357624
R8=1.07699988
A1=111.58535672
A2=105.1442642
A3=111.39304161
A4=126.50125901
A5=122.09921122
A6=132.43584095
A7=122.72942889
D1=0.
D2=0.
D3=180.
D4=180.
D5=180.
D6=180.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
Table 5. 4-Iodoimidazole: Rotational Constants (MHz), ropt = B3LYP/cc-pVTZ
|
| |
|
|
|
|
|
ropt |
Expt [1]
|
|
|
|
|
|
A
|
9529.
|
9431.8375(10)
|
|
B
|
975.
|
988.45251(12)
|
|
C
|
885.
|
894.54595(11)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
[1] G.A.Cooper, C.J.Anderson, C.Medcraft, N.Walker, J.Mol.Spectrosc. xxx,xxx(2018).
|
|
|
|
|
|
|
|
|
|
|
|
|
G.A.Cooper, C.J.Anderson, C.Medcraft, A.Legon, N.Walker, Abstract FB11, 72nd ISMS, Champaign-Urbana, Illinois, 2017.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4-Iodopyrazole
|
Iodobenzene
| 3-Iodothiophene
|
Imidazole
|
|
|
2-Iodoimidazole
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Iodine
|
|
|
|
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4Iimidazole.html |
|
|
|
|
|
|
Last
Modified 15 Sept 2018 |
|
|
|
|
|
|
|
|
|
|

