|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3-CHCl-CH2Cl |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Chlorine |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in
1,2-Dichloropropane
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
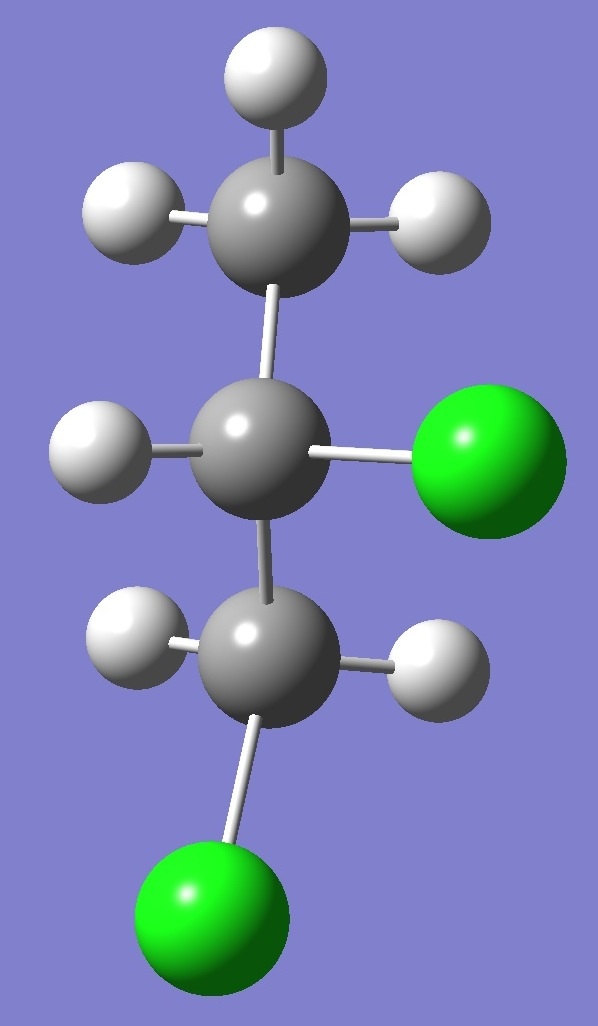
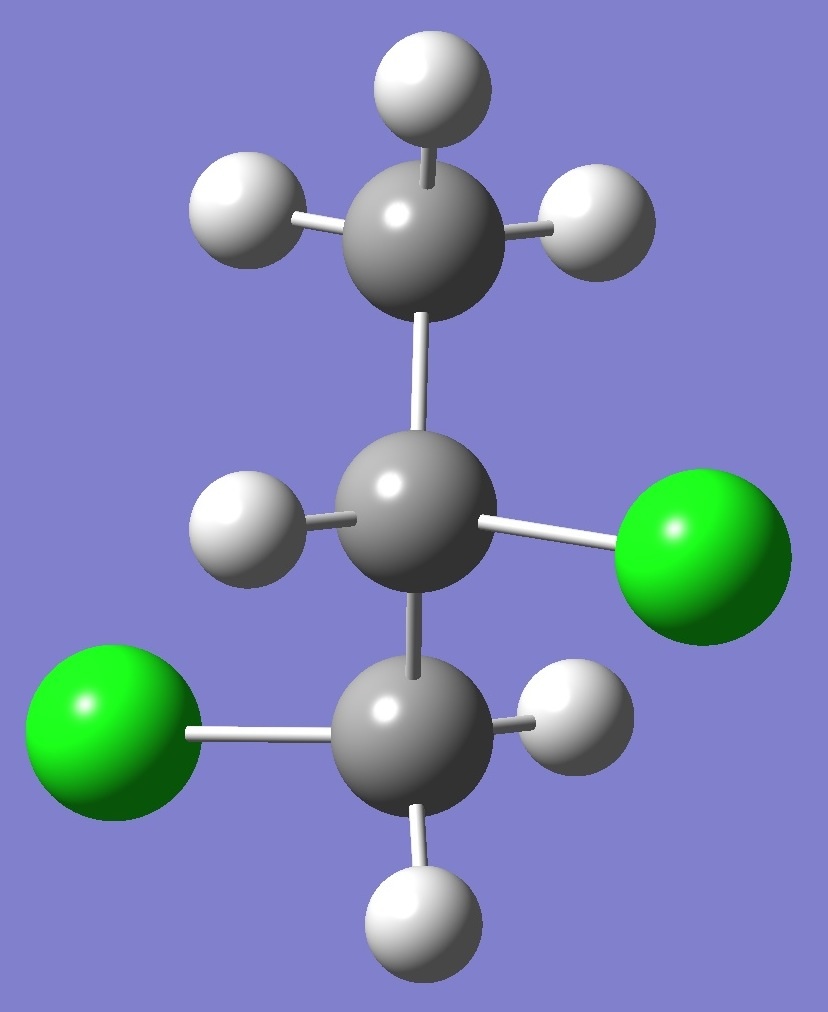
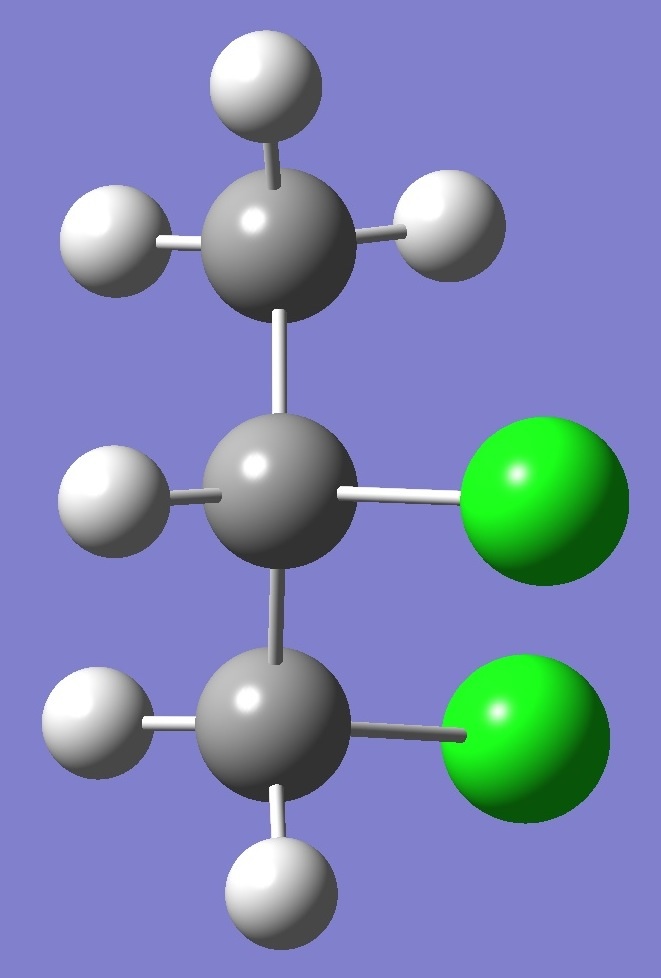
Calculation was made of the complete
nqcc tensors for 35Cl
and 37Cl
in each of three structural conformers of 1,2-dichloropropane shown
below: |
|
|
|
|
|
|
|
|
|
|
|
|
G- |
|
A |
|
G+ |
|
|
|
|
|
|
|
|
|
|
|
|
 |
|
|
|
 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results for conformer A are given on
this page. To see the results for conformers G- and
G+ click on the corresponding image.
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation was made
on MP2/6-311+G(3df,3pd) and MP2/aug-cc-pVTZ optimized structures, each
with approximate equilibrium bond lengths. Calculated nqcc
tensors are given below in Tables 1 - 5, structure parameters in
Table 6. Rotational constants and electric dipole moments are
given in Table 7. |
|
|
|
|
|
|
|
|
|
|
|
|
Energies relative to conformer A are,
for the MP2/6-311+G(3df,3pd) optimized structures, G+ =
5.50 and G- = 6.07 kJ/mole; and for
the MP2/aug-cc-pVTZ optimized structures, G+ =
5.96 and G- = 6.13 kJ/mole. |
|
|
|
|
|
|
|
|
|
|
|
|
In Tables 1 - 5, subscripts a,b,c
refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor.
Øz,CCl (degrees) is the angle between the principal
z-axis of the
nqcc tensor and the CCl bond axis. ETA = (Xxx - Xyy)/Xzz. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 1.
35Cl(10)
nqcc's in Conformer A of CH3-CH35Cl-CH235Cl(10)
(MHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd)
and (2) MP2/aug-cc-pVTZ optimized structures, each with approximate
equilibrium bond lengths. See below for atomic numbering. |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
Calc (1) |
|
Calc (2) |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
58.77 |
- |
58.59 |
|
|
|
| |
Xbb |
|
28.58 |
|
28.45 |
|
|
|
|
Xcc |
|
30.19 |
|
30.14 |
|
|
|
|
Xab |
- |
26.54 |
- |
26.62 |
|
|
|
|
Xac |
|
23.15 |
|
23.15 |
|
|
|
|
Xbc |
|
7.82 |
|
7.85 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
34.76 |
|
34.72 |
|
|
|
|
Xyy |
|
37.27 |
|
37.21 |
|
|
|
| |
Xzz |
- |
72.03 |
- |
71.92 |
|
|
|
|
ETA |
|
0.0349 |
|
0.0346 |
|
|
|
|
Øz,CCl |
|
0.31 |
|
0.31 |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 2.
35Cl(11)
nqcc's in Conformer A of CH3-CH35Cl(11)-CH237Cl
(MHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd)
and (2) MP2/aug-cc-pVTZ optimized structures, each with approximate
equilibrium bond lengths. See below for atomic numbering. |
|
| |
|
|
|
|
|
|
|
|
| |
|
|
Calc (1) |
|
Calc (2) |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
51.98 |
- |
51.77 |
|
|
|
| |
Xbb |
|
20.70 |
|
20.54 |
|
|
|
|
Xcc |
|
31.28 |
|
31.22 |
|
|
|
|
Xab |
- |
35.54 |
- |
35.61 |
|
|
|
|
Xac |
|
17.46 |
|
17.45 |
|
|
|
|
Xbc |
|
8.19 |
|
8.22 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
34.08 |
|
34.03 |
|
|
|
|
Xyy |
|
36.05 |
|
35.99 |
|
|
|
| |
Xzz |
- |
70.13 |
- |
70.03 |
|
|
|
|
ETA |
|
0.0281 |
|
0.0280 |
|
|
|
|
Øz,CCl |
|
0.66 |
|
0.63 |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 3.
Cl
nqcc's in Conformer A of CH3-CH37Cl(11)-CH235Cl(10)
(MHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd)
and (2)
MP2/aug-cc-pVTZ optimized structures, each with approximate equilibrium
bond lengths. See below for atomic numbering. |
|
| |
|
|
|
|
|
|
|
|
| |
35Cl(10) |
|
Calc (1) |
|
Calc (2) |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
59.08 |
- |
58.90 |
|
|
|
| |
Xbb |
|
28.90 |
|
28.78 |
|
|
|
|
Xcc |
|
30.18 |
|
30.13 |
|
|
|
|
Xab |
- |
26.00 |
- |
26.08 |
|
|
|
|
Xac |
|
23.22 |
|
23.21 |
|
|
|
|
Xbc |
|
7.69 |
|
7.71 |
|
|
|
|
|
|
|
|
|
|
|
|
|
37Cl(11) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa |
- |
41.30 |
- |
41.14 |
|
|
|
| |
Xbb |
|
16.66 |
|
16.53 |
|
|
|
|
Xcc |
|
24.64 |
|
24.60 |
|
|
|
|
Xab |
- |
27.65 |
- |
27.71 |
|
|
|
|
Xac |
|
13.82 |
|
13.81 |
|
|
|
|
Xbc |
|
6.38 |
|
6.40 |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 4.
Cl
nqcc's in Conformer A of CH3-CH35Cl(11)-CH237Cl(10)
(MHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd)
and (2)
MP2/aug-cc-pVTZ optimized structures, each with approximate equilibrium
bond lengths. See below for atomic numbering. |
|
| |
|
|
|
|
|
|
|
|
| |
35Cl(11) |
|
Calc (1) |
|
Calc (2) |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
51.82 |
- |
51.62 |
|
|
|
| |
Xbb |
|
20.52 |
|
20.37 |
|
|
|
|
Xcc |
|
31.30 |
|
31.25 |
|
|
|
|
Xab |
- |
35.72 |
- |
35.79 |
|
|
|
|
Xac |
|
17.38 |
|
17.37 |
|
|
|
|
Xbc |
|
8.21 |
|
8.24 |
|
|
|
|
|
|
|
|
|
|
|
|
|
37Cl(10) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa |
- |
46.24 |
- |
46.10 |
|
|
|
| |
Xbb |
|
22.42 |
|
22.32 |
|
|
|
|
Xcc |
|
23.82 |
|
23.78 |
|
|
|
|
Xab |
- |
21.09 |
- |
21.16 |
|
|
|
|
Xac |
|
18.18 |
|
18.17 |
|
|
|
|
Xbc |
|
6.20 |
|
6.22 |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Table 5.
37Cl
nqcc's in Conformer A of CH3-CH37Cl(11)-CH237Cl(10)
(MHz). Calculation was made on the (1) MP2/6-311+G(3df,3pd)
and (2)
MP2/aug-cc-pVTZ optimized structures, each with approximate equilibrium
bond lengths. See below for atomic numbering. |
|
| |
|
|
|
|
|
|
|
|
| |
37Cl(11) |
|
Calc (1) |
|
Calc (2) |
|
Expt.
|
|
| |
|
|
|
|
|
|
|
|
|
Xaa |
- |
41.18 |
- |
41.01 |
|
|
|
| |
Xbb |
|
16.51 |
|
16.39 |
|
|
|
|
Xcc |
|
24.66 |
|
24.62 |
|
|
|
|
Xab |
- |
27.81 |
- |
27.86 |
|
|
|
|
Xac |
|
13.76 |
|
13.75 |
|
|
|
|
Xbc |
|
6.40 |
|
6.42 |
|
|
|
|
|
|
|
|
|
|
|
|
|
37Cl(10) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Xaa |
- |
46.48 |
- |
46.34 |
|
|
|
| |
Xbb |
|
22.67 |
|
22.57 |
|
|
|
|
Xcc |
|
23.81 |
|
23.77 |
|
|
|
|
Xab |
- |
20.67 |
- |
20.74 |
|
|
|
|
Xac |
|
18.23 |
|
18.23 |
|
|
|
|
Xbc |
|
6.09 |
|
6.11 |
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 6. CH3-CHCl-CH2Cl,
conformer A.
Heavy atom structure parameters (Å and
degrees). r(1) = MP2/6-311+G(3df,3pd) and r(2) = MP2/aug-cc-pVTZ
optimized structures, each with approximate equilibrium bond lengths.
Complete structures are given here
in Z-matrix format. |
|
|
|
|
|
|
|
|
r(1) |
r(2) |
|
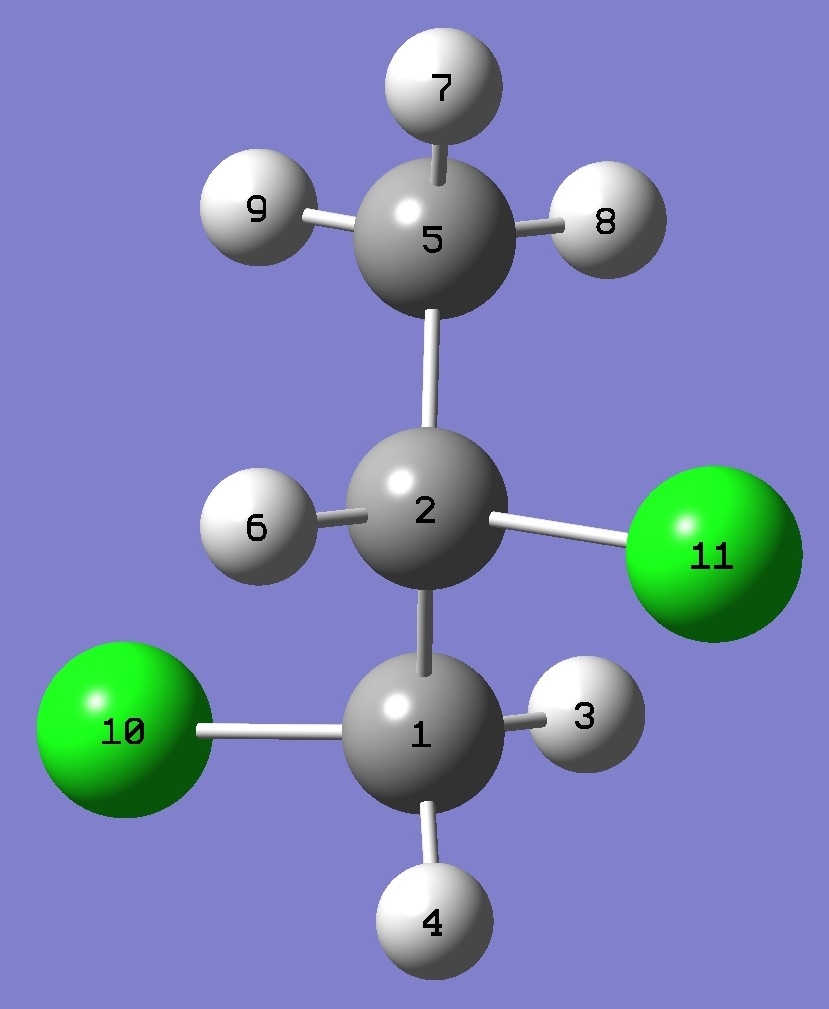 |
|
|
|
|
Cl(10)C(1) |
1.7829 |
1.7846 |
|
C(1)C(2) |
1.5142 |
1.5132 |
|
C(2)Cl(11) |
1.7944 |
1.7959 |
|
C(2)C(5) |
1.5107 |
1.5103 |
|
C(1)C(2)C(5) |
113.66 |
113.88 |
|
C(2)C(1)C1(10) |
110.37 |
110.09 |
|
C(1)C(2)Cl(11) |
106.28 |
105.99 |
|
ClC(1)C(2)Cl |
173.68 |
173.70 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
| Table 7. CH3-CH35Cl-CH235Cl,
conformer A. Rotational Constants (MHz), and Dipole Moments
(D) on the (1) MP2/6-311+G(3df,3pd) and (2) MP2/aug-cc-pVTZ
optimized structures, each with approximate equilibrium bond lengths. |
| |
|
|
|
|
| |
|
Calc (1) |
Calc (2) |
Expt. |
|
|
|
|
|
|
A |
6953.76 |
6966.22 |
|
|
B |
1485.11 |
1482.68 |
|
|
C |
1279.16 |
1277.65 |
|
|
|
|
|
|
|
|µa| |
0.01 |
0.01 |
|
|
|µb| |
0.49 |
0.49 |
|
|
|µc| |
0.07 |
0.07 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t-1-Chloropropane |
g-1-Chloropropane |
2-Chloropropane |
2,2-Dichloropropane |
|
|
2,2-Chlorofluoropropane |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
G.A.Guirgis, Y.D.Hsu, A.C.Vlaservich,
H.D.Stidham, and J.R.Durig, J.Mol.Struct. 378,83(1996). IR and
Raman. |
|
|
S.H.Schei and R.Stølevik,
J.Mol.Struct. 128,171(1985). ged |
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Chlorine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CH3CHClCH2Cl_A.html |
|
|
|
|
|
|
Last
Modified 17 Oct 2010 |
|
|
|
|
|
|
|
|
|
|



