|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
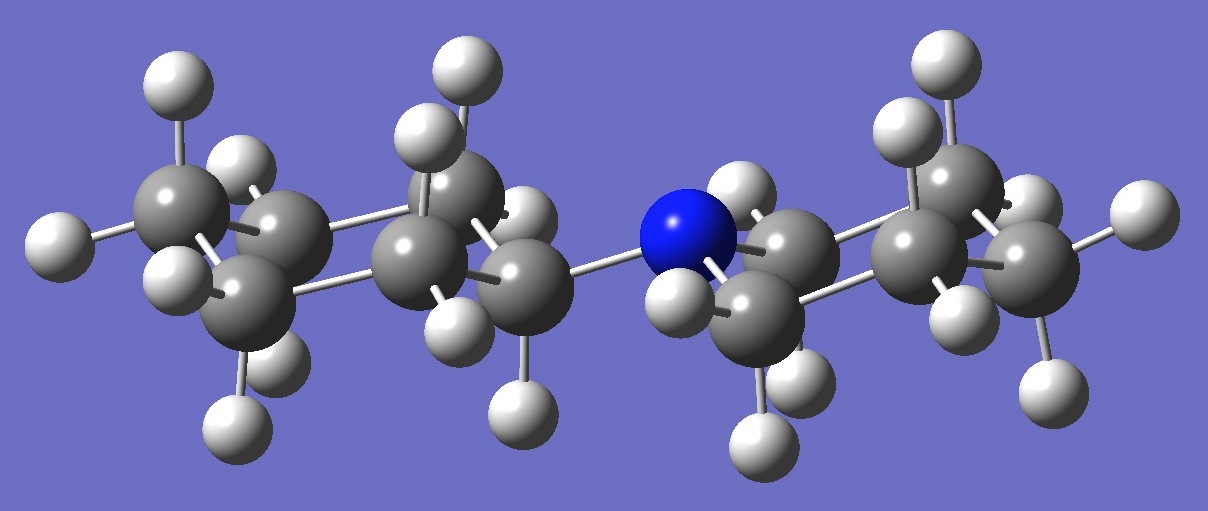
C6H11-NC5H10
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nitrogen |
|
|
|
Nuclear
Quadrupole Coupling Constants |
|
|
in N-Cyclohexyl-Piperidine
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of the 14N
nuclear quadrupole coupling constant tensor in N-cyclohexyl-piperidine was made here on an ropt molecular
structure given by B3P86/6-31G(3d,3p)
optimization (assuming Cs symmetry).
|
|
|
This
calculated nqcc tensor is given in Table 1; structure parameters in
Table 2; rotational constants and electric dipole moments in Table 4.
|
|
|
In Table 1, subscripts a,b,c refer to the
principal axes of the inertia tensor; x,y,z to the principal axes
of the nqcc tensor. ETA = (Xxx - Xyy)/Xzz. Ø (degrees) is the angle between its subscripted parameters. RSD is the calibration residual standard
deviation of the B3PW91/6-311+G(df,pd) model for calculation of the efg's/nqcc's. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
Table 1. Nitrogen nqcc's in N-cyclohexyl-piperidine (MHz). Calculation was made
on B3P86/6-31G(3d,3p) ropt structure.
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Calc
|
|
Expt
|
|
| |
|
|
|
|
|
|
|
|
14N |
Xaa |
|
2.732
|
|
|
|
|
|
Xbb |
|
2.552
|
|
|
|
|
|
Xcc |
-
|
5.284
|
|
|
|
|
|
|Xac| |
|
0.057
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RSD
|
0.030 (1.3 %)
|
|
|
|
|
|
|
|
|
|
|
|
|
Xxx |
|
2.732
|
|
|
|
|
|
Xyy |
|
2.552 |
|
|
|
|
|
Xzz |
-
|
5.284
|
|
|
|
|
|
ETA
|
-
|
0.0341
|
|
|
|
|
|
Øz,a
|
|
89.59
|
|
|
|
|
|
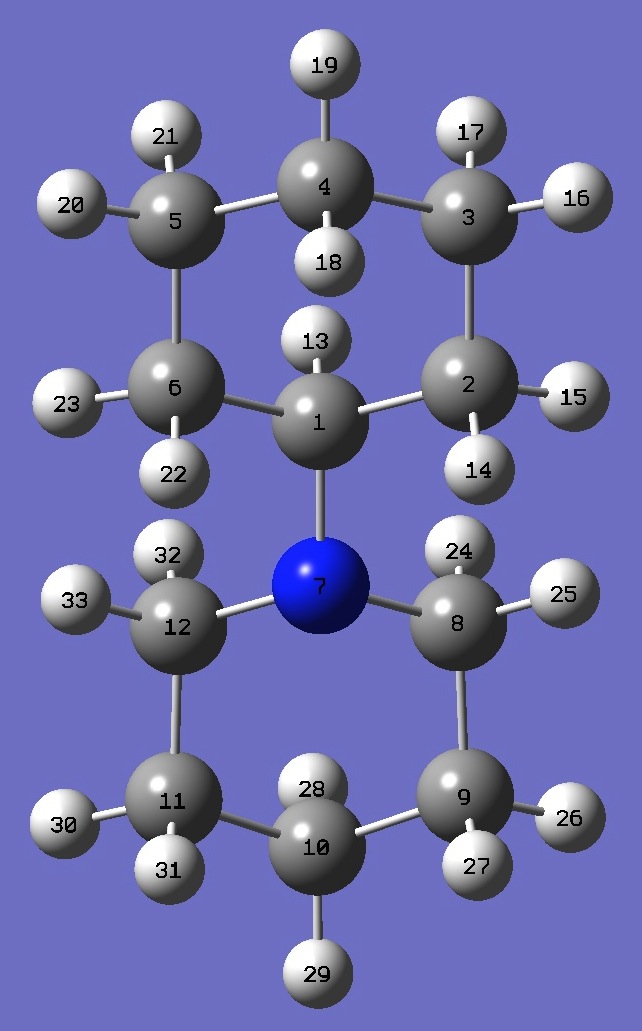
Øa,NC(1) * |
|
17.61
|
|
|
|
|
|
Øz,NC(1) *
|
|
107.20
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* See below for atomic numbering.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Table 2. N-Cyclohexyl-Piperidine. B3P86/6-31G(3d,3p) optimized structure
parameters (Å and degrees).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
# B3PW91/6-311+G(df,pd) prop scf=tight
N-Cyclohexyl-Piperidine
0 1
C
C 1 B1
C 2 B2 1 A1
C 3 B3
2 A2
1 D1 0
C 4 B4
3 A3
2 D2 0
C 5 B5
4 A4
3 D3 0
N 1 B6
2 A5
3 D4 0
C 7 B7
1 A6
2 D5 0
C 8 B8
7 A7
1 D6 0
C 9 B9
8 A8
7 D7 0
C 10 B10
9 A9
8 D8 0
C 7 B11
1 A10 2
D9 0
H 1 B12 7 A11 12 D10 0
H 2 B13
1 A12 7
D11 0
H 2 B14
1 A13 7
D12 0
H 3 B15
2 A14 1
D13 0
H 3 B16
2 A15 1
D14 0
H 4 B17
3 A16 2
D15 0
H 4 B18
3 A17 2
D16 0
H 5 B19
4 A18 3
D17 0
H 5 B20
4 A19 3
D18 0
H 6 B21
5 A20 4
D19 0
H 6 B22
5 A21 4
D20 0
H 8 B23
7 A22 1
D21 0
H 8 B24
7 A23 1
D22 0
H 9 B25
8 A24 7
D23 0
H 9 B26
8 A25 7
D24 0
H 10 B27
9 A26 8
D25 0
H 10 B28
9 A27 8
D26 0
H 11 B29 10
A28 9 D27 0
H 11 B30 10
A29 9 D28 0
H 12 B31
7 A30 1
D29 0
H 12 B32
7 A31 1
D30 0
|
|
|
|
|
|
|
|
|
|
|
|
|
B1 1.53373557
B2 1.52764031
B3 1.52209247
B4 1.52209247
B5 1.52764031
B6 1.47291301
B7 1.46010045
B8 1.52358889
B9 1.52157471
B10 1.52157471
B11 1.46010045
B12 1.11130800
B13 1.09776529
B14 1.09164339
B15 1.09472351
B16 1.09803869
B17 1.09773722
B18 1.09489346
B19 1.09472351
B20 1.09803869
B21 1.09776529
B22 1.09164339
B23 1.11010028
B24 1.08938798
B25 1.09485253
B26 1.09613499
B27 1.09790414
B28 1.09448598
B29 1.09485253
B30 1.09613499
B31 1.11010028
B32 1.08938798
A1 112.92323313
A2 111.97099103
A3 109.84934418
A4 111.97099103
A5 111.32922347
A6 112.57704148
A7 112.01641203
A8 111.24214479
A9 109.07237220
A10 112.57704148
A11 109.66049340
A12 107.99778266
A13 110.83903005
A14 109.34543960
A15 109.25621315
|
A16 109.51732322
A17 110.64110568
A18 110.76189717
A19 109.09809366
A20 109.99398327
A21 108.09004617
A22 110.82053778
A23 109.29134357
A24 108.99144127
A25 108.60524016
A26 109.46060563
A27 111.04272064
A28 111.07959874
A29 109.65448850
A30 110.82053778
A31 109.29134357
D1 56.04786252
D2 -55.07264298
D3 55.07264298
D4 -176.70056521
D5 -57.16504875
D6 174.81449216
D7 57.41287163
D8 -52.78610890
D9 178.79673428
D10 -62.01910849
D11 -54.84851495
D12 61.85268430
D13 179.19296306
D14 -64.92646015
D15 65.24582433
D16 -177.51648283
D17 177.41365510
D18 -65.99312986
D19 64.67329113
D20 -179.03655620
D21 -62.88179060
D22 54.72322713
D23 -179.77551339
D24 -63.35912965
D25 66.95183133
D26 -175.47508431
D27 174.38873698
D28 -67.36709784
D29 62.88179060
D30 -54.72322713
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table
3. N-Cyclohexyl-Piperidine. Rotational Constants (MHz) and
Electric Dipole Moments (D) calculated on B3P86/6-31G(3d,3p) optimized
structures.
|
|
|
|
|
|
|
|
Calc.
|
Expt
|
|
|
|
|
|
|
|
A
|
2255.7
|
|
|
|
B
|
495.6
|
|
|
|
C
|
425.8
|
|
|
|
|
|
|
|
|
|µa|
|
0.13
|
|
|
|
|µb| |
0 (symmetry) |
|
|
|
|µc| |
0.42
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Piperidine |
N-Methylpiperidine | 1-(1-Phenylcyclohexyl)piperidine |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table of Contents |
|
|
|
|
|
Molecules/Nitrogen |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NCychexpipe.html |
|
|
|
|
|
|
Last
Modified 25 Oct 2013 |
|
|
|
|
|
|
|
|
|
|

